All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional.
The gvhd Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the gvhd Hub cannot guarantee the accuracy of translated content. The gvhd and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The GvHD Hub is an independent medical education platform, sponsored by Medac and supported through grants from Sanofi and Therakos. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View GvHD content recommended for you
Do you know... Which of the following would indicate that a switch to second-line treatment after first-line steroids should be considered?
The GvHD Hub held a virtual symposium on October 21, 2025, titled, Guidance vs practice: How can we improve treatment of steroid-refractory graft-versus-host disease (SR-GvHD)? Here, we share a presentation by Daniel Wolff, University Hospital Regensburg, DE, discussing the early treatment strategies for SR-GvHD.
Symposium | Early treatment strategies for SR-GvHD
Symposium | Early treatment strategies for SR-GvHD
Wolff provided an overview of the treatment options for second-line treatment of GvHD and subsequent advanced-line options, emphasizing that early detection of disease progression and sufficient treatment intervention are crucial, as advanced disease is often nonreversible. Wolff discussed response assessments to first-line steroid treatment and second-line treatments, and outlined the treatment options for patients with SR-cGvHD, including ruxolitinib, belumosudil, axatilimab, ibrutinib and extracorporeal photopheresis (ECP).
Key points
- Chronic GvHD (cGvHD) is a major cause of late morbidity, occurring in 30–50% of patients who undergo allo-HSCT, and leads to significantly impaired patient quality of life (QoL).1–5
- Advanced disease is more frequently nonreversible, highlighting the importance of early detection of progression and sufficient treatment.6
- Over half of patients with cGvHD receive ≥3 lines of therapy, with decreasing efficacy. Therefore, there is a significant unmet need for novel treatments that are well tolerated and provide rapid, durable responses, as well as improve patient QoL.5,7
- At second line, treatment failure rates increase while malignancy relapse rates decline compared with first-line therapy. The cumulative incidence of successful withdrawal of immunosuppressive therapy after cGvHD resolution was 23% at 4 years with first-line treatment vs 15% with second-line therapy.8
- Response to first-line steroid treatment can indicate whether second-line treatment should be considered (Figure 1).4,9
Figure 1. Response to first-line steroid treatment indicating a switch to second-line treatment may be considered4,9
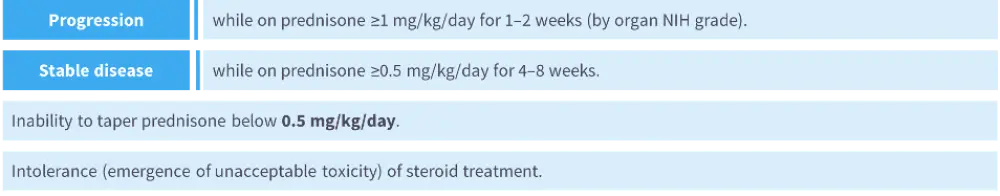
- There are various factors that may arise during assessment of patients that signal a change of treatment could be required. Examples include increases in National Institute of Health (NIH) scores (skin, eyes, and oral), loss of photographic range of motion (P-ROM) ≥1 or progression by NIH grade (fascia), or drop of forced expiratory volume in 1 second (FEV1) >10% in the absence of infection (lung).4,9,10
- Response to second-line treatment can also indicate whether third-line treatment should be considered (Figure 2).4,9,11
Figure 2. Response to second-line treatment indicating a switch to third-line treatment may be considered4,9,11

- When proceeding with third-line treatment, avoid switching more than one drug at once (except in patients showing rapid progression), in order to facilitate identification of the effective agent.4
- Wolff recommended to differentiate between cGvHD and toxicities (including infections and secondary malignancies); to assess the diagnostic symptoms of GvHD and potential concomitant diseases, with histopathologic confirmation where required; and to target cofactors (e.g. treatment of infections and avoidance of mechanical irritation in oral cGvHD).
- To monitor treatment response, NIH grading can be used to assess disease activity over time, with measurements made at diagnosis, and at 1, 3, 6, and 12 months after start of treatment.10,12,13
- Grading includes full skin assessment, oral mucosa, eyes, liver enzymes, and fascia.10,12,13
- Pulmonary function tests should be performed at diagnosis, every 3 months for the first 2 years, and after airway infection. Constrictive bronchiolitis obliterans (CBO) has a more rapid decline in FEV1 over 24 months compared with CBO with infection and lymphocytic bronchiolitis.14,15
- The P-ROM scale is a useful tool for response assessment in daily practice.16
- At diagnosis, patients should be screened for genital cGvHD, where there is an increased risk in oral cGvHD, particularly in female patients where rates vary likely due to symptom underreporting.13,17
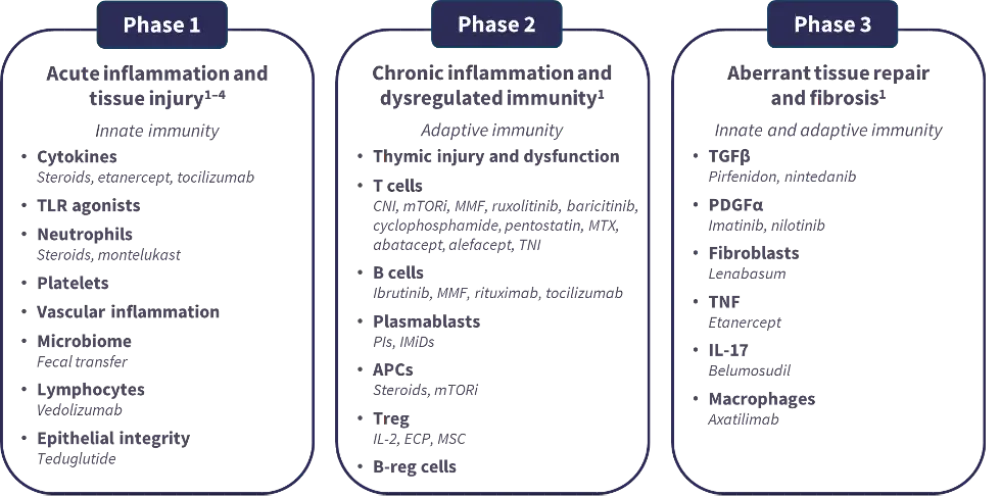
- Selection of advanced-line treatment should consider the biological phase of cGvHD, as different agents target distinct disease mechanisms (Figure 3).18–21
- When selecting an appropriate treatment, prior treatments, comorbidities, infection complications, patient compliance and expectations, drug approval status and availability, financial resources, and steroid response should also be considered.
Figure 3. Biological considerations and targets within the three phases of cGvHD18–21
Treatment options after steroids
Ruxolitinib
- Ruxolitinib is a standard of care for second-line treatment for cGvHD approved by the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA).22,23
- In the phase III REACH3 trial (NCT03112603), longer failure-free survival was observed with ruxolitinib compared with control therapy (>18.6 months vs 5.7 months; p < 0.001).
- Responses were observed across organs, with highest overall response rates (ORRs) seen in skin (52.9%), mouth (51.5%), and gastrointestinal (GI) tract (51.3%).24
- However, cytopenias and infectious complications can lead to early discontinuation of ruxolitinib, and ~40% of patients may require alternative treatments.24–26
Ruxolitinib combined with extracorporeal photopheresis (ECP)
- In the REACH3 trial, a third of patients (16/55) responded to ECP.24
- Ruxolitinib in combination with ECP demonstrated responses in patients with SR-cGvHD (N = 23) in a retrospective analysis (complete response [CR], 9%; partial response [PR], 65%). Therefore, the combination regimen may be considered for patients who respond to ruxolitinib but require additional therapies, without adding toxicity.27
Belumosudil
- Belumosudil is approved by the FDA for cGvHD after ≥2 prior lines of systemic therapy and is approved for second-line treatment of cGvHD in China.28,29
- Belumosudil demonstrated a best ORR of 76% in the phase II ROCKstar trial (NCT03640481), with high ORRs observed across organs including lower GI (72%), joints/fascia (66%), and upper GI (61%), in a pooled analysis.30,31
- Patients treated with belumosudil after ruxolitinib have a best ORR of ~45%.32
Axatilimab
- Axatilimab is approved by the FDA for treatment of cGvHD after ≥2 prior lines of therapy.33
- In the phase II AGAVE-201 trial (NCT04710576), axatilimab resulted in high rates of CR across organs including lower GI (89%), upper GI (82%), and esophagus (65%). The lowest dose of axatilimab (0.3 mg/kg) resulted in the highest response rate of 74%.34
Ibrutinib
- Ibrutinib is approved by the FDA for treatment of cGvHD after ≥1 prior lines of systemic therapy.35
- In a phase Ib/II trial (NCT02195869), a best ORR of 67% was observed with ibrutinib, with organ-specific best ORRs of 91% (GI) and 88% (skin and mouth).36
- Infectious complications, hemorrhage, cytopenias, and cardiac arrythmias have been observed with ibrutinib treatment.35
Imatinib and rituximab
- A randomized phase II crossover trial (NCT01309997) evaluated imatinib or rituximab for cutaneous sclerosis in patients with cGvHD. A significant CR was observed in 26% of patients treated with imatinib and 27% of those treated with rituximab.37
Treatment for lung GvHD after steroids
- Belumosudil, ruxolitinib, axatilimab, abatacept, and ECP are valuable treatment options in lung cGvHD.38–42
- Belumosudil resulted in a best overall improvement ≥5% from baseline in FEV1% of 50% in patients with a baseline NIH lung score of 3.39
- Patients who responded to ruxolitinib (CR or PR) had a higher predicted FEV1% compared with those who did not respond (p = 0.0001).40
- Axatilimab was associated with improvements in predicted FEV1%, with higher change from baseline observed in patients with a CR or PR.41
- Abatacept has demonstrated a particular benefit in patients with cGvHD with lung involvement.42
- As FEV1 <40% is rarely reversible, early detection and effective treatment is crucial.39,40,43
- Wolff provided a summary of his approach to treatment of SR-GvHD (Figure 4).
Figure 4. Approach to treatment of SR-GvHD

- Wolff concluded by highlighting that advanced disease is more frequently nonreversible, which calls for early detection of progression and initiation of sufficient treatment, noting that patients should also be advised on the importance of early reporting of progression and complications.
- Supportive care and screening for nonreported complications, such as distress, fatigue, and sexual dysfunction, is advised.
- A multidisciplinary team is required to address the multiorgan nature of cGvHD.
- The session concluded with a discussion and Q&A that included live audience participation.
- Wolff shared his perspectives on:
- The current treatment algorithm for SR-GvHD.
- The role of psoralen plus ultraviolet A in skin cGvHD.
- Practical approaches and indications for switching therapies.
- The role of biopsies and pathology in cGvHD.
This educational resource is independently supported by Sanofi. All content was developed by SES in collaboration with an expert steering committee. Funders were allowed no influence on the content of this resource.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
Which consideration most strongly guides your decision to escalate therapy in SR-aGvHD?


 Daniel Wolff
Daniel Wolff.webp&w=3840&q=75)
