All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional.
The gvhd Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the gvhd Hub cannot guarantee the accuracy of translated content. The gvhd and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The GvHD Hub is an independent medical education platform, sponsored by Medac and supported through grants from Sanofi and Therakos. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View GvHD content recommended for you
Symposium | Quality of life and symptoms in cGvHD
Do you know... Which statement is true regarding the PROMIS Global Health instrument?
The GvHD Hub held a virtual symposium on October 21, 2025, titled, Guidance vs practice: How can we improve treatment of steroid-refractory graft-versus-host disease (SR-GvHD)? Here, we share a presentation by Steven Pavletic, Bethesda, US, discussing quality of life and symptoms in chronic graft-versus-host disease (cGvHD).
Symposium | Quality of life and symptoms in cGvHD
Symposium | Quality of life and symptoms in cGvHD
Pavletic discussed the importance of the assessment of quality of life (QoL) using patient-reported outcome measures (PROMs) in both clinical trials and clinical practice in cGvHD, outlining the frequently used PROMs in GvHD, including cGvHD-specific and dimension-specific measures. Pavletic highlighted that incorporation of PROMs into standard of care practice may ultimately lead to more tailored and effective clinical assessments.
Key points
- The National Cancer Institute defines QoL as “the overall enjoyment of life”.1
- QoL is commonly assessed using validated multidimensional tools – such as PROMs of physical, emotional, social, and functional wellbeing – throughout the continuum of care.2
- PROMs have been shown to predict overall survival following allogeneic hematopoietic stem cell transplantation (allo-HSCT).3
- The baseline single general health question “In general, would you say your health is…” of the 36-item Short Form health survey (SF-36) was associated with overall survival, with patients replying excellent/very good/good having a hazard ratio of mortality of 0.52 compared with those answering fair or poor (p = 0.0005).4
- Randomized controlled trials indicate that incorporating PROMs into routine oncology practice can improve patient QoL and survival – an important consideration given the significant QoL burden in cGvHD.5–8
- The National Institutes of Health (NIH) 2005 consensus proposed numerous response assessments for cGvHD clinical trials, including patient-reported outcome questionnaires (Figure 1).9
Figure 1. The NIH 2005 consensus on response assessments in cGvHD9
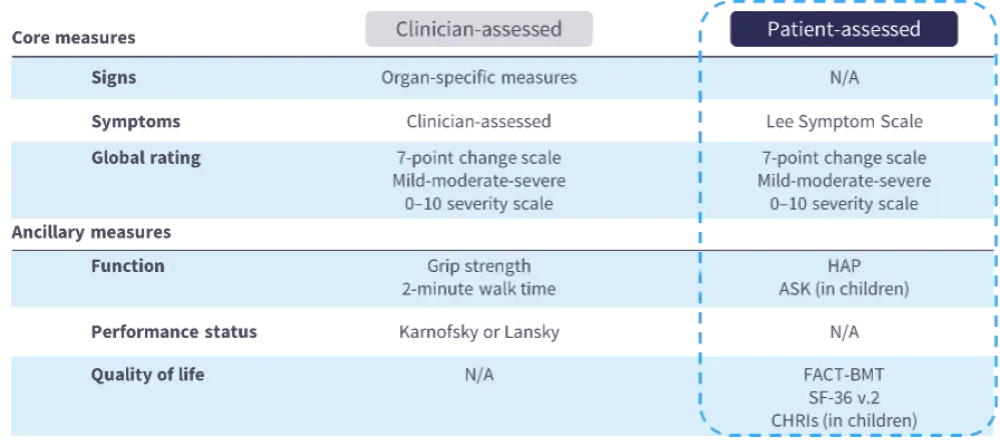
- In an NCI cohort of 189 adult patients with mild (n = 2), moderate (n = 62), or severe (n = 125) cGvHD, symptom and QoL PROMs (including 36-item Short Form health survey [SF-36] physical scale, Lee Symptom Scale, Human Activity Profile, and Functional Assessment of Cancer Therapy–Bone Marrow Transplant) were significantly associated with NIH global scores of severe cGvHD, reflecting the relevance of these measures in this setting.10
- SF-36 physical component scores in patients with moderate-to-severe cGvHD are comparable with those reported for systemic sclerosis, systemic lupus erythematosus, and multiple sclerosis, highlighting the substantial impairment in physical functioning with cGvHD.11
- Numerous PROMs have been utilized as outcome measures in studies of adult patients with GvHD, including cGvHD-specific and dimension-specific measures (Table 1).12
Table 1. Summary of PROMs used in GvHD12
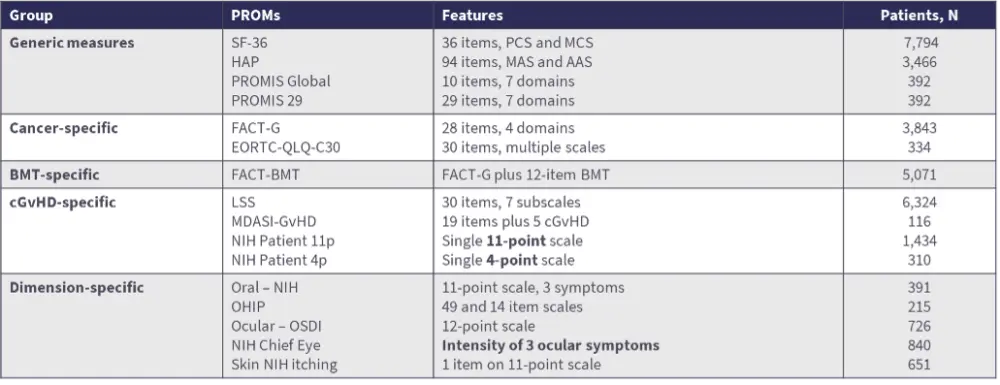
- The Lee Symptom Scale is the most frequently used PROM in cGvHD. It consists of 30 items with 7 subscales to calculate a summary score, takes approximately 2 minutes for patients to complete, and is available in 12 European languages.13–15
- Despite the approvals of ibrutinib, ruxolitinib, belumosudil, and axatilimab, complete response rates remain low, underscoring the need for better insight into patient perspectives and the magnitude of improvements.16,17
- In the phase II AGAVE-201 trial (NCT04710576), axatilimab 0.3 mg/kg resulted in CR in only 9% of patients with skin involvement (n = 64); however, 44% of patients had reduction in sclerotic skin surface area and 66% had improvement in skin tightening severity, suggesting that NIH skin organ responses inadequately capture improvements.18
- To address this limitation, the Provisional 55 items Lee Symptom Scale – Skin Sclerosis was developed as a new PRO measure with content validity for cGvHD-associated sclerosis.19
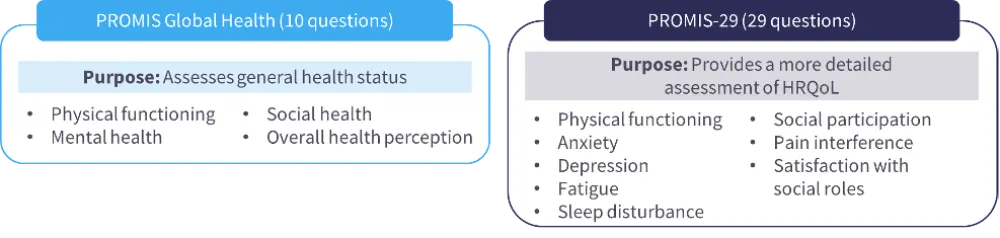
- The patient-reported outcomes measurement information system (PROMIS) offers two primary health inventories – the PROMIS Global Health and PROMIS-29 – which are publicly available without fees or license (Figure 2).20
Figure 2. The PROMIS Global Health and PROMIS-29 measures20
- The Patient-Reported Outcomes version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE) captures symptoms in aGvHD effectively, though challenges such as limited health literacy, acute and concurrent illness, frequent reminders, and research/personnel support can impact survey completion rates.21
- Ibrutinib demonstrated Lee Symptom Scale results that complemented clinical efficacy in the first regulatory approval trial for refractory cGvHD.17,22
- Overall response was correlated with the LSS summary score in patients treated with belumosudil in clinical trials.23
- In the AGAVE-201 trial, 60% of patients treated with axatilimab 0.3 mg/kg had a clinically meaningful reduction in cGvHD symptoms (>5-point reduction in the modified LSS score).18
- The patient’s perspective is increasingly important to assess. A cGvHD patient survey identified the top concerns that patients want addressed in their care, including management of stamina and fatigue, cognitive effects (memory and attention), disease progression, and available treatment options.24
- A study by the cGvHD Consortium showed that 71% of patients perceived cGvHD improvement at 6 months; however, patient-reported response had limited correlation with clinician-reported (κ 0.37) or NIH cGvHD response (κ 0.18), highlighting that PROs are an important complementary endpoint in cGvHD trials and drug development.25
- A community advisory board with GvHD patient advocates, established to better understand the unmet needs of patients with GvHD in Europe, outlined five actionable themes: the need for reliable and tailored information, increased patient empowerment, access to professional dedicated care, optimal emotional support, and attention to the financial implications of GvHD, with improved communication as an overarching theme.26
- The patient perspective is not routinely evaluated in clinical practice using validated PROMs. Incorporation of this into standard of care practice may ultimately lead to more tailored and effective clinical assessments.2,27
- Future directions include:
- Validating PROs in cGvHD clinical trials, as proposed by the American Society for Transplantation and Cellular Therapy.
- Incorporating PROMs into clinical care and generating evidence-based data that guide interventions.
- Expanding patient self-reports to include areas less commonly addressed, such as occupational therapy and lifestyle medicine pillars.
- Integrating objective and subjective measures into new holistic assessment tools for cGvHD disease activity and response.
- The session concluded with a discussion and Q&A that included live audience participation, where Pavletic shared his perspectives on the role of PROs and QoL assessments for informing treatment switching and selection.
This educational resource is independently supported by Sanofi. All content was developed by SES in collaboration with an expert steering committee. Funders were allowed no influence on the content of this resource.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

