All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional.
The gvhd Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the gvhd Hub cannot guarantee the accuracy of translated content. The gvhd and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The GvHD Hub is an independent medical education platform, sponsored by Medac and supported through grants from Sanofi and Therakos. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View GvHD content recommended for you
Symposium | The importance of real-world data in allogeneic stem cell transplantation: A focus on cGvHD
Featured:
Do you know... Which statement is true regarding the use of real-world data (RWD)?
The GvHD Hub held a virtual symposium on October 21, 2025, titled, Guidance vs practice: How can we improve treatment of steroid-refractory graft-versus-host disease (SR-GvHD)? Here, we share a presentation by Mohamad Mohty, Sorbonne University Hôpital Saint-Antoine, Paris, FR, discussing the importance of real-world data (RWD) in allogeneic stem cell transplantation with a focus on chronic GvHD (cGvHD).
Symposium | The importance of real-world data in allogeneic stem cell transplantation: A focus on cGvHD
Symposium | The importance of real-world data in allogeneic stem cell transplantation: A focus on cGvHD
Mohty provided an overview of RWD, discussing their role in regulatory decision-making, the various types of RWD, study designs, and limitations. Mohty explored whether pivotal clinical trial results can be reproduced with RWD, highlighting the consistent outcomes observed in a French compassionate use program of belumosudil. Mohty concluded the session by providing a patient case focusing on treatment selection in SR-GvHD.
Key points
- RWD are data relating to patient health status and/or healthcare delivery, which are routinely collected from a variety of sources. Real-world evidence (RWE) is clinical evidence on the use and potential benefits of a medical product, derived from analysis of RWD.1
- RWD/RWE are clinically relevant for physicians and payers; they can allow for hypothesis generation, comparative effectiveness, treatment strategy assessments, quality of care, and effectiveness.2,3
- In regulatory decision-making, RWD/RWE can enable analysis of real-world drug use patterns, post-authorization safety studies, detection of drug–drug interactions, and economic modeling.2–5
- They can also assess alternative dosing regimens for established medicines, identify target–drug combinations for randomized controlled trials, and support label expansion.2,3,6
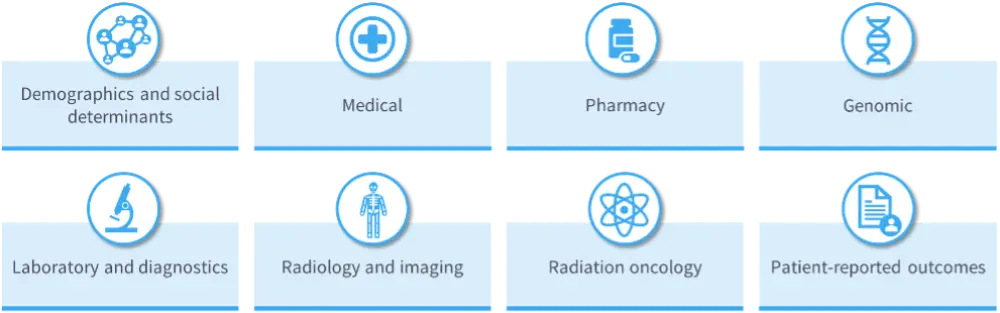
- Numerous types of RWD can be collected across many settings (Figure 1), which can be merged or aggregated, and collected longitudinally.3,7
Figure 1. Types of RWD3
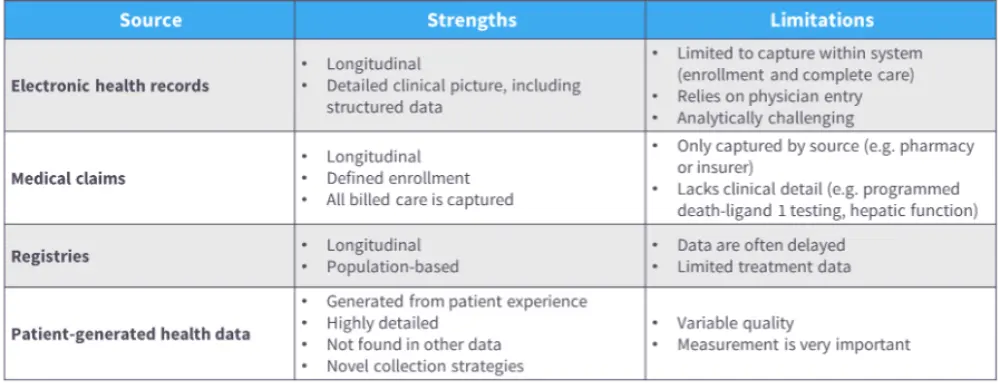
- For selecting an appropriate data source for RWD/RWE, various strengths and limitations can be considered (Table 1).
Table 1. Strengths and limitations of different data sources2,3,8
- RWD/RWD study designs include pragmatic clinical trials, prospective and retrospective observational studies, externally controlled trials, and descriptive studies.3,9,10
- Limitations of RWD/RWE include missing data or availability of key data, completeness of available variables, lack of randomization and written protocols, and lack of monitoring and control over the quality of data collection.3,11,12
- RWD can enhance understanding of approved drugs and reproduce clinical trial outcomes in real-world settings, strengthening confidence in their clinical use.
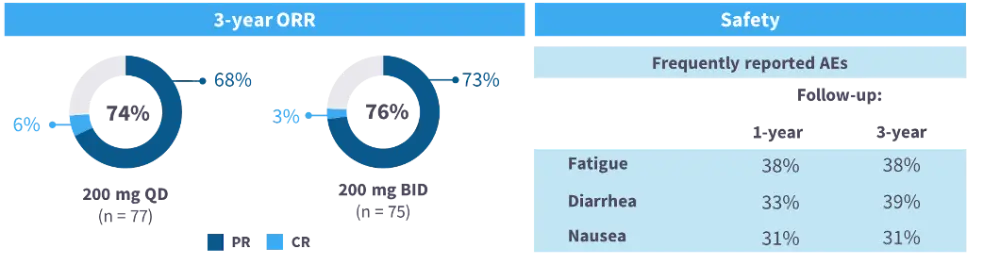
- Belumosudil, a Rho-associated protein kinase 2 inhibitor, was evaluated in the multicenter, phase II ROCKstar trial (NCT03640481) in patients with cGvHD who had received 2–5 prior lines of therapy. The best overall response rate (ORR; primary endpoint) at 1 year was 74% and 77% for the 200 mg once daily (n = 66) and 200 mg twice daily (n = 66) dose arms, respectively.13
- The 3-year follow-up of the ROCKstar study demonstrated consistent efficacy and tolerability of belumosudil (Figure 2).14,15
Figure 2. 3-year ORR and frequently reported AEs with belumosudil from the ROCKstar study13–15
- In a French compassionate use program, 68 patients with cGvHD received belumosudil, the majority (94%) of whom had moderate–severe cGvHD and a median (range) of 3 (2–8) treatment lines prior to belumosudil at onset.16
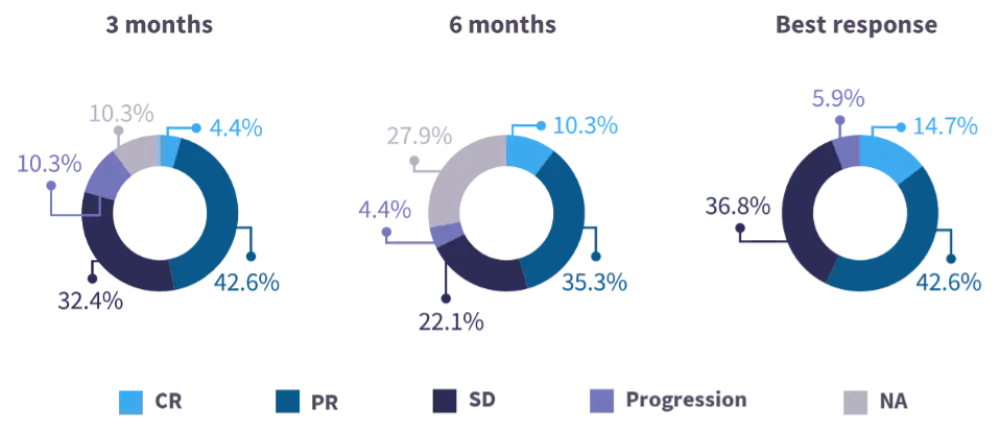
- After a median follow-up of 337 days, belumosudil responses were observed at 3 and 6 months, with 57% of patients experiencing a complete response (CR) or partial response (PR) as their best response (Figure 3).16
Figure 3. Real-world response data with belumosudil from a French compassionate use program16
- Overall survival rates at 6 and 12 months were 89.1% and 84.7%, respectively. Failure-free survival rates at 6 and 12 months were 89.1% and 80.4%, respectively.16
- Nine patients died on treatment, predominantly from GvHD, and ten patients experienced AEs (Table 2).16
Table 2. Cause of death and AEs with belumosudil from a French compassionate use program16
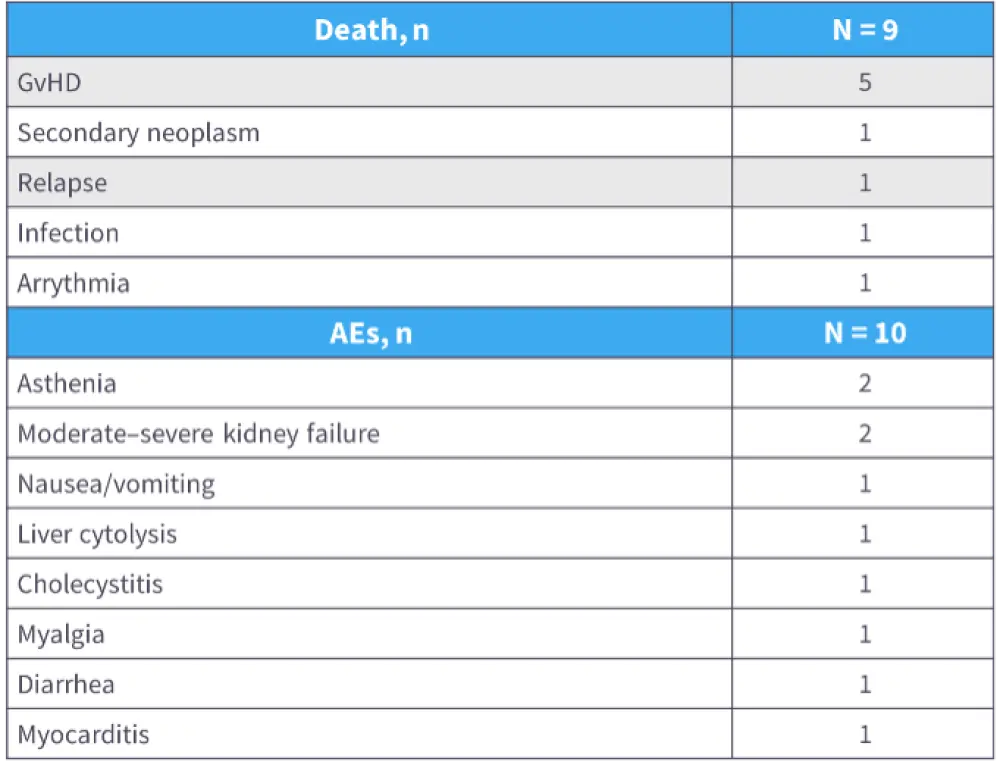
- Overall, results of this French compassionate use program of real-world belumosudil were comparable with previous clinical trials.16
Patient case study
- Mohty presented a patient case of a female aged 49 years with acute myeloid leukemia in first complete remission, who underwent a peripheral blood allogeneic hematopoietic stem cell transplantation and went on to develop SR-GvHD.
- The next-step management options discussed included ruxolitinib, extracorporeal photopheresis, and belumosudil, though treatment options may depend on country-specific approvals and individual labels.
- Mohty concludes by summarizing that RWD can provide unique insights into patient journeys, treatment strategies, and outcomes, highlighting that they complement clinical trials through assessment of effectiveness and safety in more diverse patient populations.
This educational resource is independently supported by Sanofi. All content was developed by SES in collaboration with an expert steering committee. Funders were allowed no influence on the content of this resource.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content


 Mohamad Mohty
Mohamad Mohty