All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional.
The gvhd Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the gvhd Hub cannot guarantee the accuracy of translated content. The gvhd and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The GvHD Hub is an independent medical education platform, sponsored by Medac and supported through grants from Sanofi and Therakos. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View GvHD content recommended for you
PTCy, tacrolimus, and mycophenolate mofetil as GvHD prophylaxis
At the 64th American Society of Hematology (ASH) Meeting and Exposition, Holtan1 presented a late-breaking abstract on the phase III BMT CTN 1703 trial (NCT03959241), which we are pleased to provide a summary of here.
The trial investigated the novel combination of posttransplant cyclophosphamide (PTCy), tacrolimus (Tac), and mycophenolate mofetil (MMF) for graft-versus-host disease (GvHD) prophylaxis, compared with the standard of care of Tac and methotrexate (MTX). The novel combination used in this study was selected as it was the best-performing novel combination in the previous BMT CTN 1203 trial.
Study design
The BMT CTN 1703 trial enrolled 431 patients across 37 centers in the US, who received reduced-intensity conditioning and peripheral blood stem cell grafts. The primary endpoint of this study was 1-year GvHD relapse-free survival (defined as Grade 3–5 GvHD, chronic GvHD that required systemic immunosuppression, relapse/progression of disease, or death). Patients were randomized 1:1 to either PTCy/Tac/MMF or TAC/MTX.
Results
Baseline patient characteristics are shown in Table 1. The majority of patients had acute myelogenous leukemia as their primary disease and received a transplant from an unrelated donor.
Table 1. Baseline patient characteristics*
|
HLA, human leukocyte antigen; MMF, mycophenolate mofetil; MTX, methotrexate; PTCy, posttransplant cyclophosphamide; Tac, tacrolimus. |
||
|
Characteristic, % (unless otherwise stated) |
PTCy/Tac/MMF |
Tac/MTX |
|---|---|---|
|
Sex |
|
|
|
Male |
62.6 |
58.1 |
|
Median age (range), years |
66.1 (20.1–78.6) |
66.3 (26.3–77.4) |
|
Primary disease |
|
|
|
Acute lymphoblastic leukemia |
5.6 |
12.4 |
|
Acute myelogenous leukemia |
50.0 |
46.1 |
|
Biphenotypic leukemia |
0.5 |
0.5 |
|
Chronic myeloid leukemia |
2.8 |
2.3 |
|
Myelodysplastic syndrome |
29.4 |
30.0 |
|
Lymphoma (all subtypes) |
10.7 |
7.8 |
|
Donor type and HLA matching |
|
|
|
Related donor 6/6 |
28.0 |
31.3 |
|
Unrelated donor 7/8 |
3.3 |
3.7 |
|
Unrelated donor 8/8 |
68.7 |
65.0 |
|
Conditioning regimen |
|
|
|
Fludarabine/busulfan |
26.2 |
28.1 |
|
Fludarabine/melphalan |
57.0 |
56.7 |
|
Other |
14.0 |
13.4 |
|
Not transplanted |
2.8 |
1.8 |
Outcome measures
- The primary endpoint of 1-year GvHD relapse-free survival was found to be significantly higher in patients receiving PTCy/Tac/MMF (52.7%) compared with patients receiving Tac/MTX (34.9%).
- There were ten secondary outcomes measured. Among the secondary outcomes, GvHD-related outcomes are shown in Figure 1, with other outcomes shown in Figure 2.
- Cumulative incidence of chronic GvHD at 1-year was significantly higher in patients treated with Tac/MTX than in patients treated with PTCy/Tac/MMF. Conversely, the cumulative incidence of Grade 2 infections at 12 months was significantly higher in the PTCy/Tac/MMF arm.
Figure 1. Disease-free survival and GvHD outcomes*
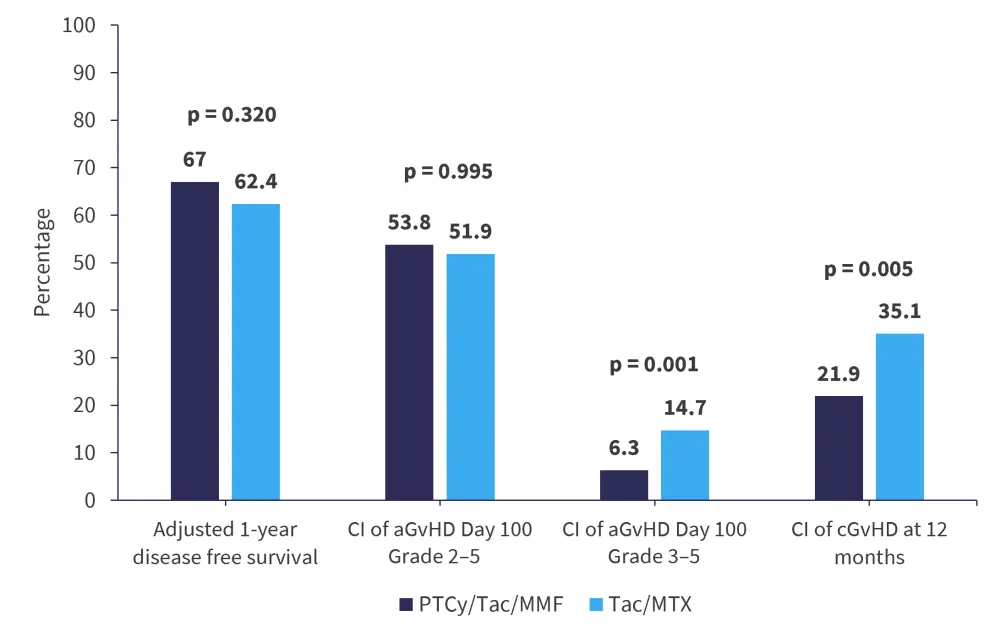
aGvHD, acute graft-versus-host disease; cGvHD, chronic graft-versus-host disease; CI, cumulative incidence; MMF, mycophenolate mofetil; MTX, methotrexate; PTCy, posttransplant cyclophosphamide; Tac, tacrolimus.
*Adapted from Holtan.1
Figure 2. Other secondary outcome measures*
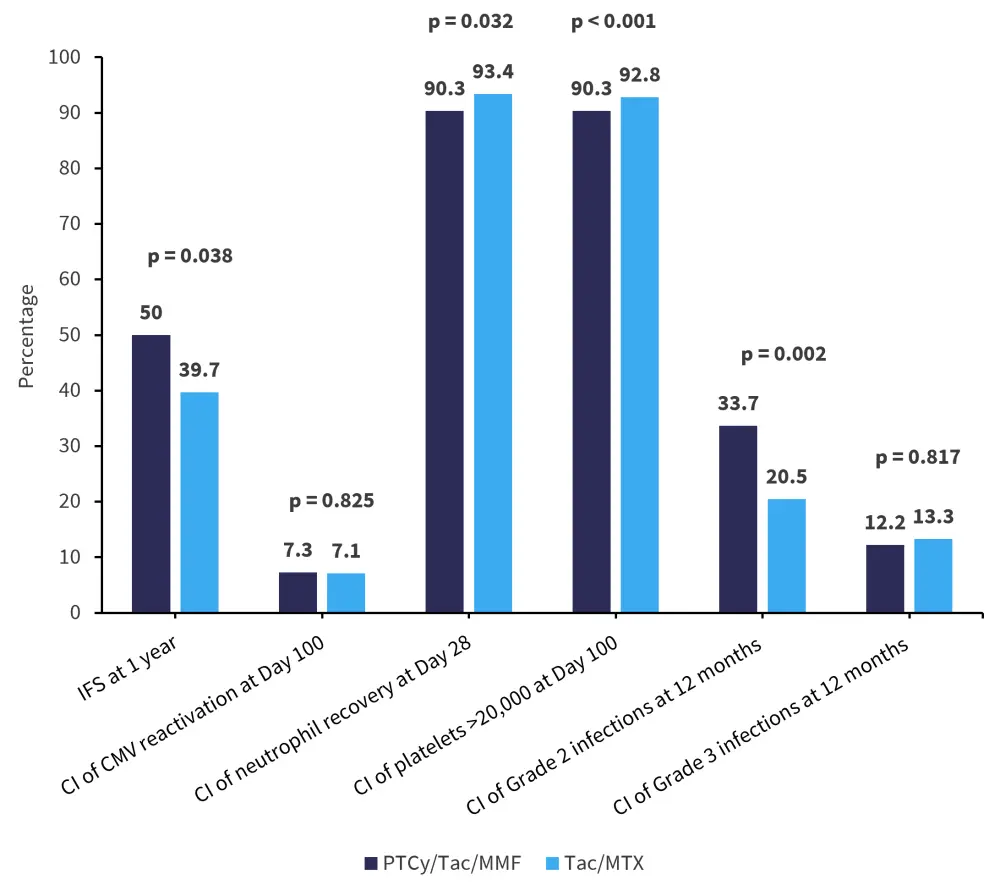
aGvHD, acute graft-versus-host disease; CI, cumulative incidence; CMV, cytomegalovirus; IFS, immunosuppression-free survival; MMF, mycophenolate mofetil; MTX, methotrexate; PTCy, posttransplant cyclophosphamide; Tac, tacrolimus.
*Data from Holtan.1
There were no differences in chimerism or rejection in the trial, with a graft rejection rate of 3% in the PTCy/Tac/MMF arm and 0.5% in the Tac/MTX arm. The most common cause of death was recurrent or persistent disease, affecting 39.6% of patients in the PTCy/Tac/MMF arm and 42.9% of patients in the Tac/MTX arm. Acute GvHD was a more common cause of death in the Tac/MTX arm (14.3%) compared with the PTCy/Tac/MMF arm (4.2%), whereas organ failure was a common cause of death in the PTCy/Tac/MMF arm (22.9%) compared with the Tac/MTX arm (10.7%).
Conclusion
Use of PTCy/Tac/MMF as GvHD prophylaxis produced a superior 1-year GvHD relapse-free survival compared with the current standard of care, due to reducing the occurrence of acute and chronic GvHD. Although treatment with PTCy/Tac/MMF increased Grade 2 infections, it did not cause an increase in Grade 3 infections, and the majority of infections occurred within the first month. Disease relapse was found to be similar across the arm; thus, Holtan concluded that PTCy/Tac/MMF should become the new standard of care in adult patients who are receiving reduced-intensity conditioning transplantation. Further analysis of patient-reported outcomes and microbiota studies are planned.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
Which consideration most strongly guides your decision to escalate therapy in SR-aGvHD?



