All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional.
The gvhd Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the gvhd Hub cannot guarantee the accuracy of translated content. The gvhd and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The GvHD Hub is an independent medical education platform, sponsored by Medac and supported through grants from Sanofi and Therakos. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View GvHD content recommended for you
Do you know... In the REACH3 trial, which included patients aged ≥12 years with moderate or severe glucocorticoid-refractory or dependent cGvHD, ORR was assessed for nine best available therapies. Which of the following reflects the order of therapies with the top four overall response rates?
Video series
On November 29, 2022, the GvHD Hub held a virtual symposium on the role of extracorporeal photopheresis (ECP) in the management of graft-versus-host disease (GvHD).
Here, we share the third presentation, given by Professor Daniel Wolff, University Hospital Regensburg, Regensburg, D,E which looked at the future use of ECP in patients with GvHD.
In this presentation, Professor Wolff discusses the current status of treatment in GvHD and assesses what is in store for the treatment of acute GvHD (aGvHD) and chronic GvHD (cGvHD) with ECP. Professor Wolff outlines methods to explore patient biological profiles to establish predictive biomarkers and discusses urgent questions for future research into ECP (Figure 1).
Figure 1. Urgent questions for future research into ECP*
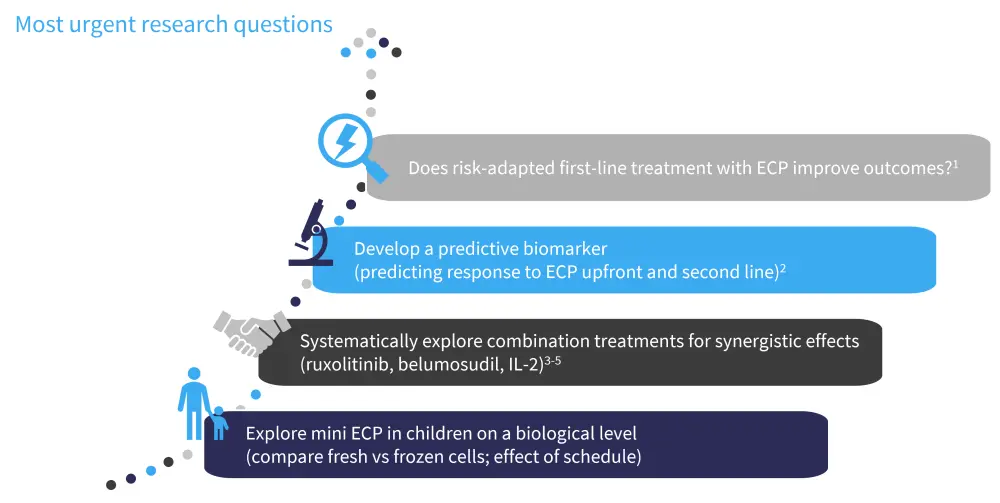
ECP, extracorporeal photopheresis; IL, interleukin.
*Data from ClinicalTrials.gov1–2; Belizaire, et al.3; and Maas-Bauer, et al.4; and Zeiser, et al.5
Watch or download the presentation to learn more about the future of ECP, including:
- The current status of aGvHD treatment (00:17; slide 4)
- The current status of cGvHD treatment (01:55; slide 5)
- The outlook for the treatment of aGvHD (03:19; slide 6)
- The outlook for the treatment of cGvHD (05:48; slide 7)
- The outlook for the future of ECP (07:26; slides 8−9)
Key points
- aGvHD and cGvHD both currently have a poor prognosis, with high rates of long-term treatment-related mortality.
- In aGvHD, there is ongoing research into risk-adapted first-line treatment with upfront ECP and steroids for high-risk patients.
- ECP may be an option for patients with aGvHD or cGvHD who do not respond to ruxolitinib or ECP may be used in combination with other immunosuppressants.
- ECP may be appropriate for patients at high risk of infectious or cytopenic complications, which is a contraindication for the use of ruxolitinib.
- Further research into patients’ biological profiles and response to treatment may help to develop predictive biomarkers for ECP.
This independent educational activity was supported by Mallinckrodt Pharmaceuticals. All content was developed independently by the faculty. The funder was allowed no influence on the content of this activity.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content


 Daniel Wolff
Daniel Wolff

