All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional.
The gvhd Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the gvhd Hub cannot guarantee the accuracy of translated content. The gvhd and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The GvHD Hub is an independent medical education platform, sponsored by Medac and supported through grants from Sanofi and Therakos. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View GvHD content recommended for you
Biomarkers for optimizing ECP treatment for acute and chronic GvHD
Do you know... Which of the following biomarker patterns is most consistently associated with ECP response in GvHD?
Graft-versus-host disease (GvHD) is a leading cause of non-relapse mortality (NRM) following allogeneic hematopoietic stem cell transplantation (allo-HSCT).1–3 Acute GvHD (aGvHD) is predominantly inflammatory, typically involving the skin, gastrointestinal (GI) tract, and liver, whereas chronic GvHD (cGvHD) has autoimmune and fibrotic features with multisystem involvement. Contemporary classification emphasizes clinical manifestations rather than time from transplant.3,4 aGvHD is associated with substantial morbidity and mortality; severe (Grade 3–4) disease shows ~36% mortality, with lower-GI involvement driving higher NRM and infections, compounding risk.1,3 cGVHD is the leading cause of late NRM, leading to multisystem functional impairment with reduced quality of life and increasing risks of serious infection and secondary malignancy.1,2 Management of GvHD aims to suppress donor–cell alloreactivity without eroding graft-versus-leukemia effects, while managing toxicities and infection risk.1,2,4 Corticosteroids remain the standard first-line therapy for both aGvHD and cGvHD. Second-line management includes immunosuppressive agents for steroid-refractory (SR) or steroid-dependent disease; however, there is a risk of long-term immunosuppression, limited response rates, and treatment-related side effects.2–4
Extracorporeal photopheresis (ECP) is an immunomodulatory therapy (Figure 1) that minimizes broad, cytopenia-inducing immunosuppression, shows efficacy in both aGvHD and cGvHD, and is generally not associated with increased post-transplant relapse or infection risk.2,4 Prospective and retrospective series demonstrate meaningful response rates in SR aGvHD and cGvHD, with rapid responses in aGvHD and high organ-specific activity in cGvHD.4,5 However, ECP is logistically complex and requires patients to be clinically stable. Growing evidence supports early biomarker use to risk-stratify patients and predict response to ECP, guiding both timing of initiation and duration of therapy.4,5
Figure 1. Mechanism of action of ECP*
.webp)
Biomarkers in aGvHD and cGvHD
Biomarkers are being evaluated to improve diagnosis, risk stratification, and treatment guidance in GvHD. The Biomarkers, EndpointS, and other Tools (BEST) glossary and framework – created by the National Institutes of Health and U.S. Food and Drug Administration (NIH-FDA) – standardize biomarker definitions and their roles (diagnostic, prognostic, predictive, response, risk) in research and practice. Many biomarker candidates have been identified; however, few are fully validated for routine clinical decision-making.4,6
In aGvHD (Table 1), soluble proteins such as soluble suppression of tumorigenicity-2 (ST2) and regenerating islet-derived 3 alpha (REG3A; and their combination in the MAGIC Algorithm Probability [MAP]) repeatedly associate with NRM severity, while TIM3, sTNFR1, and organ-specific markers (e.g. elafin for skin; REG3A/TIM3 for lower GI) add diagnostic and prognostic nuance, and may support earlier response assessment.4,6
Table 1. Biomarkers in aGvHD*
Biomarker / panel | Subtype (BEST) | Timing / context | Key signal / association | Practical use |
|---|---|---|---|---|
IL-2Rα + HGF + IL-8 + sTNFR1 | Diagnostic (systemic) | At symptom onset | Confirms systemic aGvHD with high diagnostic accuracy | Support diagnosis when clinical picture is unclear |
REG3A | Diagnostic (lower GI); prognostic | At GI symptom onset; Days +7–28 post-HSCT | Distinguishes lower-GI aGvHD; higher levels associated with higher-grade GI disease and increased NRM | Differentiate GI aGvHD from mimics; early risk stratification |
TIM3 (soluble) | Diagnostic (GI); prognostic / response | Day +28; during steroid therapy | Elevated in GI aGvHD; with ST2, higher levels ~Day 14 of steroids predict treatment failure and higher NRM | Early on-treatment check to guide escalation |
Elafin | Diagnostic (skin) | At rash onset | Elevated at skin aGvHD onset; specificity limited in some cohorts | Adjunct for cutaneous involvement; interpret with clinical context |
ST2 | Prognostic; predictive (therapy-specific) | Days +14/+28 post-HSCT; at steroid start | High ST2 predicts increased NRM and steroid resistance; replicated across settings | Baseline/early risk; anticipate steroid non-response |
MAP | Prognostic algorithm; response biomarker | Day +7 post-HSCT; also, after steroid start | Stratifies 6-month NRM; higher scores predict death independent of clinical response; validated across cohorts | Early risk grouping; complements clinical assessment |
sTNFR1 | Predictive / prognostic | Early post-HSCT; on-treatment | Associates with severe aGvHD and 1-year NRM; included in response-prediction models | Augment prediction of severe disease/NRM |
aGvHD, acute graft-versus-host disease; BEST, Biomarkers, EndpointS, and other Tools; GI, gastrointestinal; HSCT, hematopoietic stem cell transplantation; HGF, hepatocyte growth factor; IL-2Rα, interleukin-2 receptor alpha; IL-6, interleukin-6; IL-8, interleukin-8; MAP, MAGIC Algorithm Probability; NRM, non-relapse mortality; REG3A, regenerating islet-derived 3 alpha; sTNFR1, soluble tumor necrosis factor receptor 1; ST2, soluble suppression of tumorigenicity-2; TIM3, T-cell immunoglobulin and mucin domain-containing protein 3. | ||||
In cGvHD (Table 2), B-cell activating factor (BAFF), C-X-C chemokine ligands 9 and 10 (CXCL9/10), and a validated 4-protein panel (ST2, CXCL9, matrix metalloproteinase [MMP-3], osteopontin) demonstrate diagnostic and prognostic signals, including correlations with severity and NRM.4,6
Table 2. Biomarkers in cGvHD*
Biomarker / panel | Subtype (BEST) | Timing / context | Key signal / association | Practical use |
|---|---|---|---|---|
BAFF | Diagnostic / disease activity; risk (BAFF/B-cell ratio) | Early post-HSCT (~3 months) and at onset | Elevated at onset/active disease; abnormal BAFF/B-cell ratios at ~3 months predict later cGvHD | Early flag for impending/active cGvHD; monitor B-cell reconstitution context |
CXCL9 | Diagnostic / disease activity | New-onset; 6–9 months | Elevated at onset and on follow-up; associated with cGvHD across cohorts | Track inflammatory activity; potential severity signal |
CXCL10 | Diagnostic / disease activity | Replication cohorts | Elevated at onset; replicated association (viral infection can confound levels) | Complement CXCL9 for activity assessment |
4-protein panel (ST2, CXCL9, | Diagnostic; prognostic | Day +100 and at diagnosis | Correlates with diagnosis, severity, and NRM; Day +100 values predict near-term cGvHD; AUC up to ~0.89 in verification | Cohort-level risk stratification; candidate for clinical triage pending validation |
MMP-3 (individual) | Diagnostic | At onset of BOS symptoms | Higher in BOS vs controls; contributes to the 4-protein panel biology | Potential non-invasive blood test for BOS diagnosis |
AUC, area under the curve; BAFF, B-cell activating factor; BEST, Biomarkers, EndpointS, and other Tools (NIH–FDA); BOS, bronchiolitis obliterans syndrome; cGvHD, chronic graft-versus-host disease; CXCL10, C-X-C motif chemokine ligand 10; CXCL9, C-X-C motif chemokine ligand 9; HSCT, hematopoietic stem cell transplantation; MMP-3, matrix metalloproteinase-3; NRM, non-relapse mortality; ST2, suppression of tumorigenicity-2. | ||||
Biomarkers in ECP
Many of the biomarkers identified in GvHD pathophysiology also have relevance in the context of ECP, either as baseline predictors of disease biology or as pharmacodynamic (PD) indicators of response.4,5 While ECP is an effective therapy, it can be logistically difficult and requires patients to be sufficiently stable.4 There is also variability between different treatment centers in patient selection and in timing, intensity, and duration of treatment.8
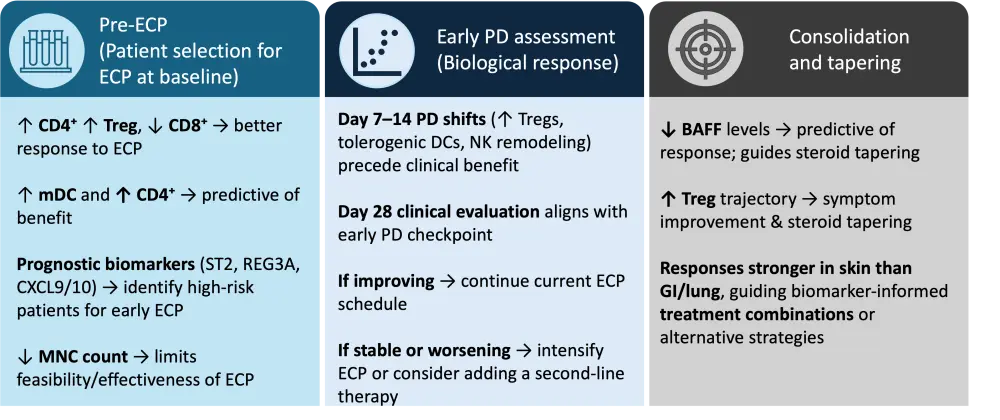
Biomarkers have the potential to reduce this variability and guide more effective treatment by helping to determine which patients are most likely to respond, how early biological effects can be detected, and to inform treatment consolidation and de-escalation.4,5 Only a subset of the broader GvHD biomarker repertoire, particularly those reflecting immune regulation, such as BAFF, regulatory T cells (Tregs), dendritic-cell subsets, and natural killer (NK) cell profiles, appear to hold promise for ECP-specific decision-making.9–11 Accordingly, biomarker use can be organized into three practical checkpoints: baseline selection, early on-treatment PD assessment, and consolidation/taper (Figure 2).5,9–11
Baseline selection of patients pre-ECP
At baseline, a higher CD3⁺CD4⁺ proportion with Treg enrichment, and a lower CD3⁺CD8⁺ proportion, has been found to be associated with better response to ECP, whereas broad soluble cytokine panels were not discriminatory.5 In cGvHD, baseline myeloid dendritic cell (mDC) counts ≥3.7/µL and CD4⁺ T-cell counts ≥104/µL predicted response and superior survival.8 In addition, severe cytopenias can limit the feasibility and effectiveness of ECP as mononuclear cell counts below 200 cells/µL yield insufficient lymphocytes and monocytes for an adequate photoactivated product, often necessitating treatment delay or modification.8
Beyond established biomarkers, several small studies have explored additional experimental biomarkers (e.g. clonal T-cell patterns, B-cell subset ratios, and circulating microRNAs) that may relate to ECP response, though these remain unvalidated and are not yet clinically applicable.2,4
Finally, established prognostic GvHD biomarkers, such as ST2, REG3A, and CXCL9/10, may help identify patients with high-risk trajectories, supporting earlier consideration of ECP in those most likely to benefit.3,6
Early on-treatment PD assessment
Rather than relying on isolated biomarker values, tracking the trajectory of immune-cell changes during the early treatment phase provides more meaningful insight into ECP response. In a study performed by Amat et al.,5 immune monitoring at Days 7 and 14 after ECP initiation captured early pharmacodynamic shifts that preceded clinical improvement, while formal clinical evaluation was performed at approximately Day 28. Similarly, Whittle and Taylor11 showed that BAFF levels at 1 month predicted 3- and 6-month outcomes and corticosteroid tapering. Together, these findings support the concept of an early pharmacodynamic checkpoint within the first 2–4 weeks of therapy, when biological evidence of response can be assessed to guide treatment continuation or adjustment.
At this stage, characteristic immunological shifts begin to emerge. Patients who respond to ECP typically show increasing Tregs and tolerogenic dendritic-cell features2,4,5 as well as a decrease in CD56-bright cytotoxic NK cells, maturation of CD56-dim NK cells, and an increase in CD57 and preserved antiviral/antileukemic NK cells.9 PD responses combined with clinical benefit can guide ECP treatment schedule and the need for additional or alternative treatments.4,5
Use of biomarkers to guide consolidation and taper
During the consolidation phase of ECP, several biomarkers can help inform decisions around treatment tapering and long-term disease control. In skin-predominant cGvHD, lower BAFF levels (<4 ng/mL at 1 month) have been shown to predict clinical response or complete remission at 3–6 months, supporting corticosteroid tapering, whereas persistently elevated BAFF is associated with poorer outcomes.11 Over extended courses, a rising trajectory of circulating Tregs often parallels symptomatic improvement and steroid reduction, indicating PD evidence of disease control.12 ECP responses are typically more pronounced in skin involvement and less robust in severe gastrointestinal or pulmonary disease, indicating where biomarker-informed treatment combinations or alternative therapeutic strategies may be most beneficial.13
Figure 2. Biomarker-guided ECP pathway*
Conclusion
In recent years, significant progress has been made in understanding the immunopathology of GvHD and the mechanisms by which ECP modulates immune responses. As insights continue to move from bench to bedside, there is growing potential to harness immunological features of GvHD to improve diagnosis, monitoring, and treatment. In the context of ECP, this includes identifying and validating biomarkers that reflect biological response and clinical outcomes. A deeper understanding of the mechanism of action of ECP, through its effects on dendritic cells, regulatory T cells, and NK-cell subsets, can support the development of biomarker-informed approaches to guide patient selection, treatment intensity and duration, and decisions about tapering or combining with other therapies. Further research is needed to determine how best to incorporate GvHD biomarkers into ECP treatment pathways, with the goal of personalizing care, minimizing toxicity, and maximizing clinical benefit for each patient.
This educational resource is independently supported by Therakos. All content was developed by SES in collaboration with an expert steering committee. Funders were allowed no influence on the content of this resource.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
Which consideration most strongly guides your decision to escalate therapy in SR-aGvHD?





