All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional.
The gvhd Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the gvhd Hub cannot guarantee the accuracy of translated content. The gvhd and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The GvHD Hub is an independent medical education platform, sponsored by Medac and supported through grants from Sanofi and Therakos. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View GvHD content recommended for you
ECP for the treatment of cGvHD: Emerging data and clinical management considerations
Do you know... According to studies by Sniecinski et al. and Abu-Dalle, ECP is particularly efficacious in which manifestation of GvHD?
In chronic graft-versus-host disease (cGvHD), immunosuppressant corticosteroids remain a first-line treatment, despite producing sustained responses in only 40–50% of patients.1 Steroid treatment duration can be prolonged, with a median duration of 2–3 years, but this can lead to long-term side effects such as infection, hyperglycemia, and osteopenia.1 Furthermore, there is a lack of clear evidence as to the most appropriate treatments second-line and beyond for cGvHD, with treatment selection also influenced by physician preference, financial situation of the patient, and availability of therapies.1
Overview of ECP in the treatment of cGvHD
Extracorporeal photopheresis (ECP) is an immunomodulatory apheresis procedure that is recommended as a second-line (or later) treatment for cGvHD.2 ECP may be favored due to its immunomodulatory, rather than immunosuppressive mode of action, in addition to being steroid-sparing, which can reduce infection risk.2 The development of ECP originated from PUVA therapy, where psoralen is used to sensitize the skin and patients are then exposed to ultraviolet-A light; this therapy is now mainly used in dermatological conditions.3
The U.S Food and Drug Administration (FDA) approved ECP in 1988, when it was used mainly for Sezary syndrome.3 It was not investigated in GvHD until 1994,3 with the first prospective, randomized trial of ECP for cGvHD published in 2008 by Flowers et al.4 Since then, there has been extensive research into ECP for GvHD in adult patients; however, there is a lack of clinical trial data supporting its use in pediatric patients, with current recommendations based on retrospective data in this population.5
ECP: Procedure and mechanism of action
Whole blood is collected from patients via either the peripheral vein or a permanently implanted catheter, and then centrifuged to remove mononuclear cells (Figure 1).3,6 After this, mononuclear cells are treated with 8-methoxypsoralen (8-MOP) and exposed to ultraviolet-A light (Figure 2).3 During this stage, three main processes occur7:
- 8-MOP induced apoptosis
- monocyte-to-dendritic cell differentiation and cytokine profile
- T-cell subpopulation modifications
Patients are then reinfused with the irradiated cells. There are two methods to carry out this process; the closed, in line-method, and the open, off-line method.3 The closed method involves only one step, with cell-separation, 8-MOP infusion, photoactivation, and re-infusion performed within one specialized device.3 In the open method, cell-separation is carried out in a separate device to the reinfusion.3
Figure 1. The ECP procedure*
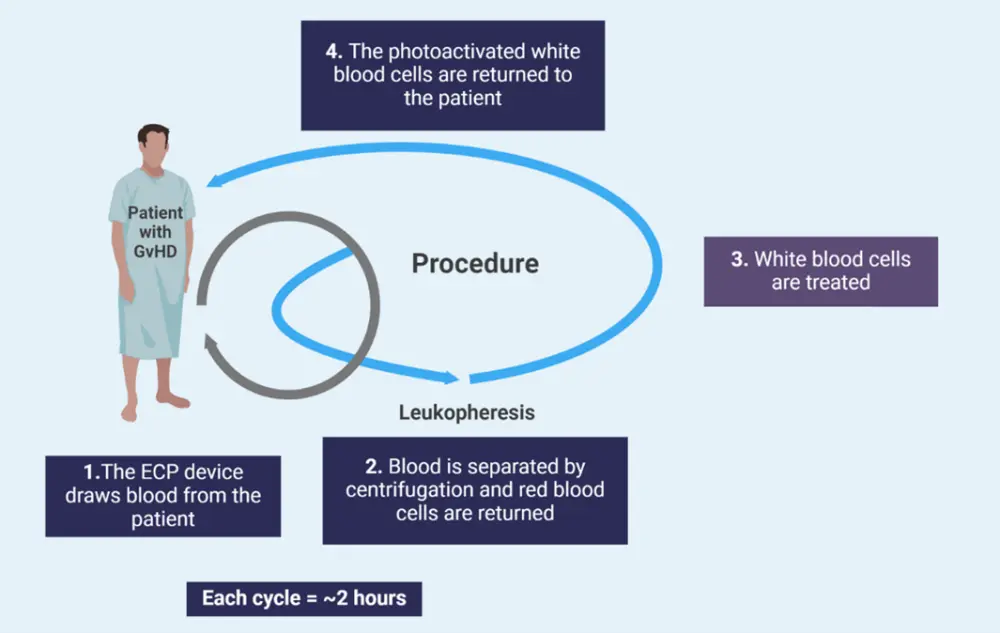
ECP, extracorporeal photopheresis; GvHD, graft-versus-host disease.
*Adapted from Drexler, et al.3 and Asensi Cantó P, et al.7 Created with BioRender.com.
Figure 2. The mechanism of action of ECP*
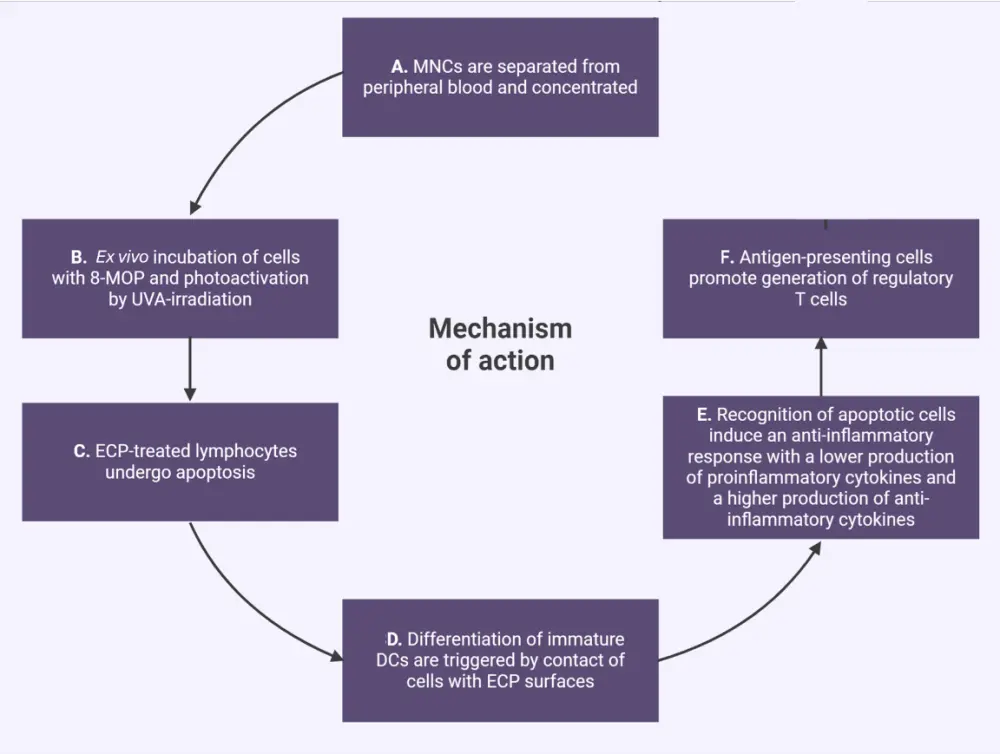
DC, dendritic cell; ECP, extracorporeal photopheresis; 8-MOP, 8-methoxypsoralen; MNC, mononuclear cell; UVA, ultraviolet-A.
*Adapted from Drexler, et al.3 and Asensi Cantó P, et al.7 Created with BioRender.com.
Question 1 / 1
Which of the following statements about the process of ECP is false?
A
Exposure to 8-MOP and photoactivation with ultraviolet-A light triggers apoptosis of mononuclear cells
B
It results in modifications to T-cell subpopulations, with generation of regulatory T cells
C
The original cellular cytokine profile is retained
D
Monocyte-to-dendritic cell differentiation occurs following contact with ECP surfaces
ECP as first-line treatment
Although ECP is generally recommended as a second-line (or later) treatment, several studies have investigated ECP as a first-line treatment in cGvHD. A prospective, randomized study by Jagasia et al.8 investigated standard-of-care treatment (SoC) versus SoC with ECP for 26 weeks in 60 patients with cGvHD.8 In the intention-to-treat population, overall response (OR) was higher in the SoC + ECP group versus SoC-only group when using blinded assessment (Figure 3).8
Figure 3. Response rates to SoC + ECP vs SoC*
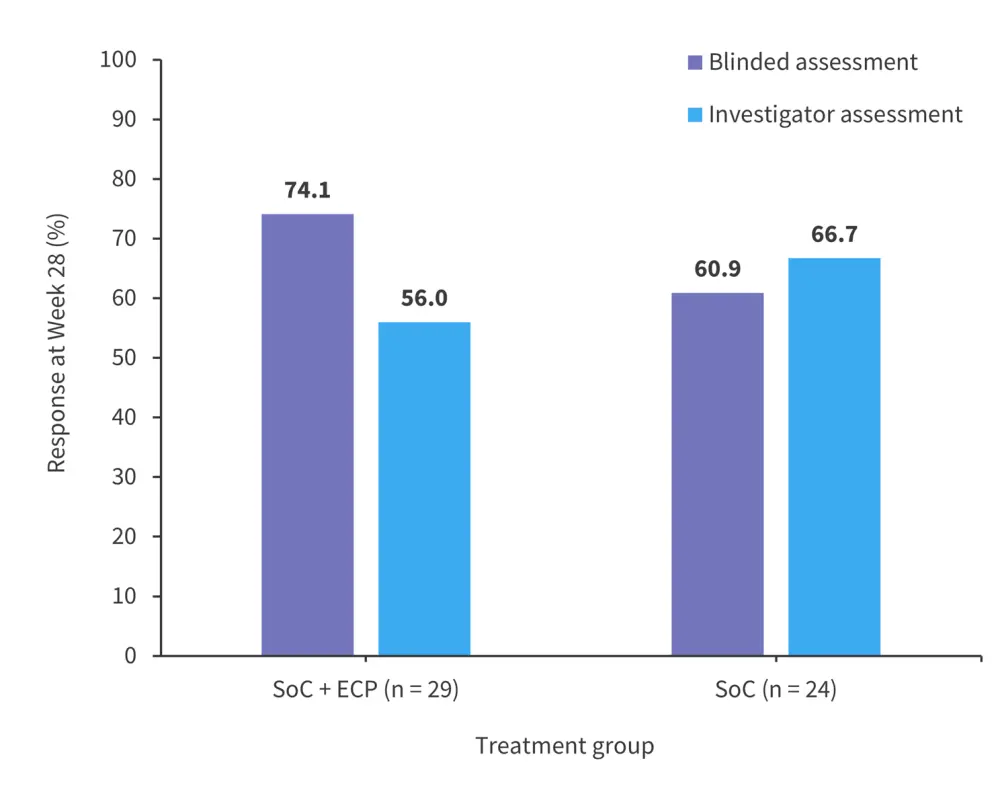
ECP, extracorporeal photopheresis; SoC, standard-of-care.
*Adapted from Jagasia, et al.8
Overall, 96.6% of SoC + ECP-treated patients and 90.3% of SoC-treated patients experienced treatment-emergent adverse events.8 The total number of adverse events was lower in the SoC + ECP arm (n = 223) compared with the SoC arm (n = 316).8 These results suggest that ECP could be beneficial in some patient populations for the treatment of cGvHD as a first-line alternative to steroids. However, further randomized, double-blinded larger trials are needed to support these findings and to inform which patients may have the greatest benefit from ECP as a first-line therapy.
ECP as second-line or later treatment
In the first prospective, randomized study of ECP for cGvHD, patients with corticosteroid-refractory cGvHD were randomized 1:1 to receive ECP + SoC, or just SoC for 12 weeks, with a primary endpoint of median change in total skin score (TSS) at Week 12 vs baseline.4 In the modified intention-to-treat population, at Week 12, patients treated with ECP had a greater median change from baseline in TSS compared with SoC only, although this difference was not significant (Table 1). Serious adverse events occurred in 28.6% of patients treated with ECP and 26% of patients in the control arm in the 12-week comparison period.4
Table 1. TSS and corticosteroid response to ECP*
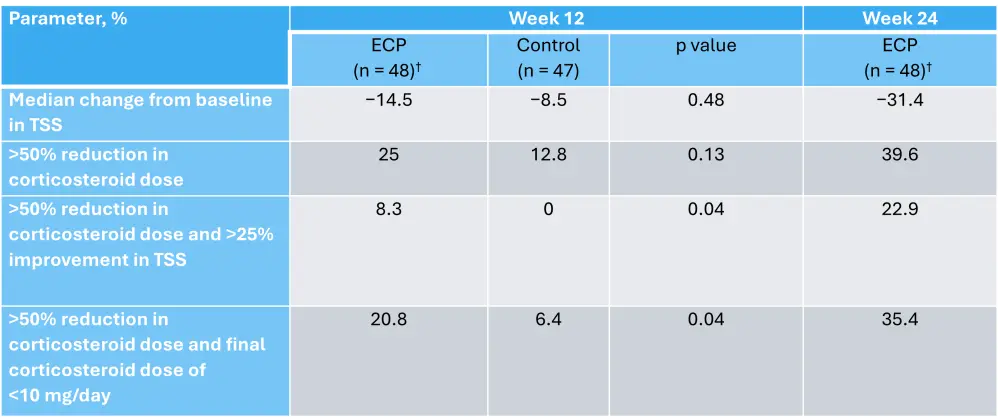
ECP, extracorporeal photopheresis; TSS, total skin score.
*Adapted from Flowers, et al.4
†In the ECP group, 41 patients were receiving treatment with corticosteroids at baseline and 38 had doses recorded at Week 12. In the control group, 43 patients were receiving treatment with corticosteroids at baseline and 39 had doses recorded at Week 12.
ECP has also been shown to be effective in patients with cGvHD who have been heavily pretreated with steroids.9 In a retrospective analysis of 75 patients who received ECP for cGvHD between 2007 and 2021, the partial response rate was 22% at 3 months, which increased to 63% at 12 months. In total, 91% of patients had severe cGvHD and 98% had previously received prednisone. By 12 months post-starting ECP, 64% of patients had successfully discontinued steroids.9
A recent observational study of 81 patients with steroid-refractory cGvHD (SR-cGvHD) who received ECP as a second-line therapy found that 78% were responders.10 Responding patients were more likely to have skin manifestations, while non-responding manifestations were more likely to involve organs, especially the liver and gut. Median overall survival was 72 months and patients who were responders to ECP had a significant difference in survival compared with ECP non-responders.10
ECP is a well-established second-line therapy option in cGvHD and has an excellent safety profile, with a similar incidence of adverse events compared with SoC treatments.4 It has demonstrated particularly good efficacy in treating skin manifestations of cGvHD,4,10 and has also been used successfully in patients with severe disease9; therefore, ECP should be considered as a possible treatment for the majority of patients with cGvHD.
Question 1 / 1
Patients with SR-cGvHD who respond to ECP second-line are more likely to have which manifestation?
A
Eye
B
Gut
C
Skin
D
Liver
ECP-based combinations
ECP has also been investigated in combination with other treatments. In a retrospective analysis by Maas-Bauer et al.11 ECP was combined with ruxolitinib (ECP-Rux) in 23 patients with cGvHD who had failed multiple previous therapies, including steroids. Most patients received ECP-Rux in addition to other therapy. At 24 months, overall survival was 75%, and overall response rate was 74% after ≥1 week of ECP-Rux. The most common adverse events were cytomegalovirus reactivation (26%) and newly diagnosed cytopenia (22%).11
Another treatment that has been investigated as a combination with ECP is interleukin-2 (IL-2).12 A phase II study enrolled 25 patients with a median of two prior systemic cGvHD therapies. Patients were treated with ECP alone, then a combination of ECP and IL-2. After 8 weeks of standalone ECP, the majority of patients had stable disease (Table 2). Objective partial response increased after an additional 8 weeks of ECP with IL-2. One patient discontinued due to adverse events after 3 weeks of treatment with IL-2. In patients who remained in the study, none required an IL-2 dose adjustment.12
Table 2. Responses after 8 and 16 weeks of ECP treatment*
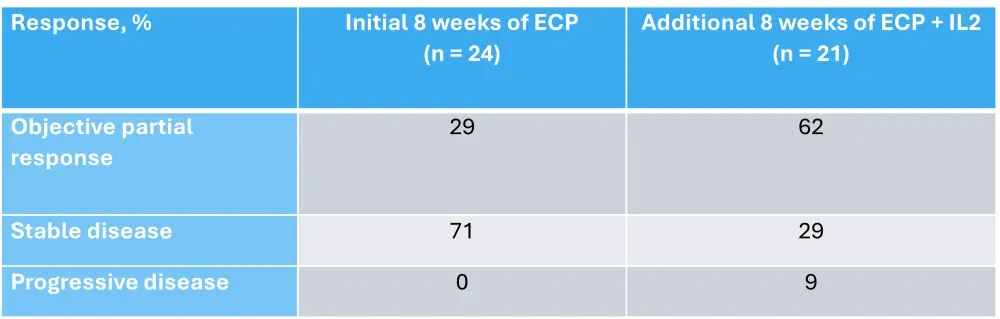
ECP, extracorporeal photopheresis; IL-2, interleukin-2.
*Data from Belizaire, et al.12
These data suggest that in patients with cGvHD who have failed previous therapies, combining ECP with other treatments may lead to better outcomes, including disease stabilization and increased survival. However, additional studies are needed to explore optimal combinations and dosing, especially when considering the variation of disease severity and manifestations across patients with cGvHD. New novel agents are likely to be discovered in the future and could further improve outcomes when combined with ECP.
Clinical considerations
ECP scheduling can vary, from multiple treatments per week, to prolonged treatment intervals of once every 4–6 weeks.13 There has been no confirmed association between intensity of ECP dosing and response.13 However, in patients with SR-GvHD, higher response rates have been noted when ECP was administered earlier in the course of disease.13 Therefore, ECP should be initiated as soon as possible once the indication is confirmed in order to improve outcomes.13 Recommendations suggest that ECP should be performed on 2 consecutive days every 1–2 weeks for ≥8 weeks, but ideally until there is a response to treatment.3
Response can vary depending on manifestation and GvHD phenotype.13 A systematic review by Abu-Dalle et al.14 found that response rates with ECP varied across manifestations, with the highest OR of 71% in cutaneous and the lowest OR of 45% in musculoskeletal manifestation (Figure 4). Other studies have reported similar findings. A study by Sniecinski et al.15 reported an OR of 80% in cutaneous, 88% in oral, 42% in liver, and 33% in lung manifestations among 26 patients.15 Overall, studies suggest higher response rates in cutaneous and oral cGvHD.14 Therefore, when selecting a treatment in patients with cGvHD, it is important to select optimal treatment based on GvHD severity and manifestation, taking sequencing of other therapies into account. Patients with severe skin involvement may experience a higher-than-average response to ECP, therefore it is especially important to consider ECP in these patients.
Figure 4. Response rates to ECP across cGvHD manifestations*
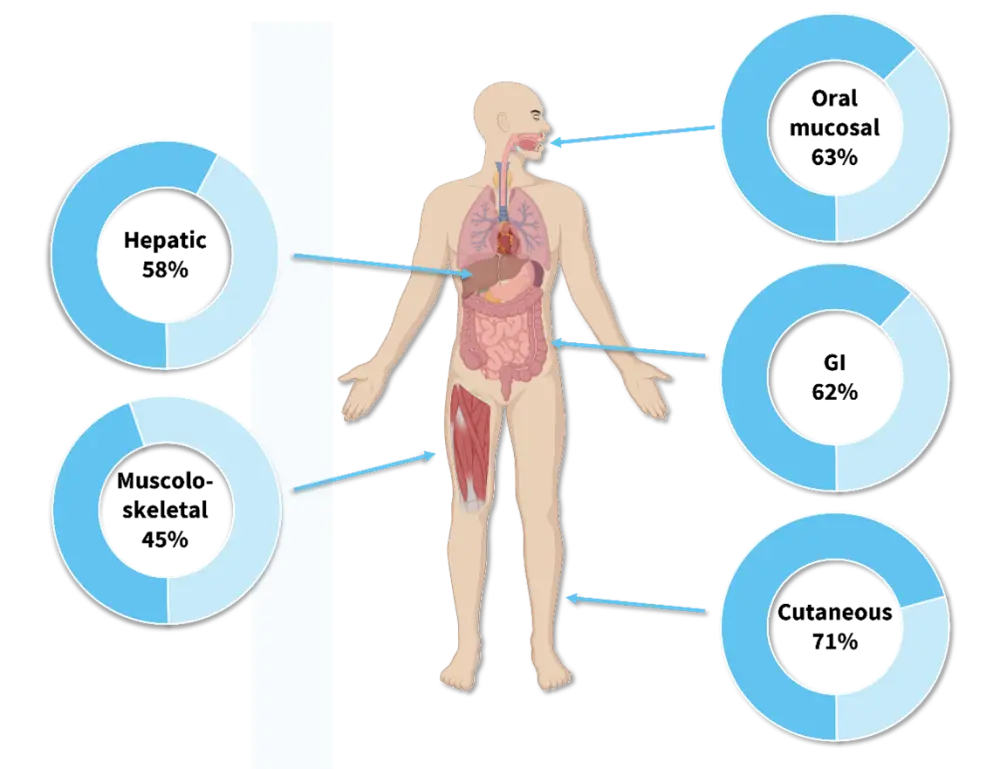
cGvHD, chronic graft-versus-host disease; ECP, extracorporeal photopheresis; GI, gastrointestinal.
*Data from Abu-Dalle et al.14 Created with Biorender.com.
Although ECP has an excellent safety profile, management of patients who have undergone ECP requires extra consideration. One of the main challenges of ECP is reliable vascular access, which is essential to ensure blood flow throughout the procedure, with difficulties including low flow rate and blood clots.16
After undergoing ECP, patients should wear extra skin and eye protection for the first 24 hours following the treatment and be observed for issues resulting from the venous access, such as hematoma, infection, and thromboembolism.3 Patients with hemodynamic instability, concurrent uncontrolled infection, or coagulation disorders should not be considered for ECP treatment. 3 Similarly, ECP is contraindicated in patients with an intolerance to methoxsalen.3
In the pediatric setting, the main challenge in using ECP is low patient weight. ECP requires a high extracorporeal volume, causing limitations for its use in pediatric patients.16 However, extracorporeal volumes have been successfully reduced to <200 ml in recent years for low-body-weight children, but may require additional considerations in the management of the procedure.16
Question 1 / 1
Which of the following statements is true?
A
Reliable vascular access is required for ECP, as blood flow is essential throughout the procedure
B
All of the above
C
Patients should wear extra skin and eye protection for the first 24 hours following ECP
D
ECP should be performed on 2 consecutive days every 1–2 weeks for ≥8 weeks (until there is a response to treatment
E
ECP should be initiated as soon as possible once SR-GvHD is confirmed
Conclusion
The data summarized here demonstrate the efficacy of ECP in patients with cGvHD, both after steroid failure and as a first-line therapy. ECP appears to be particularly efficacious in cutaneous GvHD, with overall responses of ~70–80%.13,15 Another benefit of ECP is its steroid-sparing effect, with studies showing that after treatment with ECP, over 60% of patients are able to stop steroids.9 However, there are still insufficient data to inform an optimal treatment regimen for ECP, particularly when considering combination therapies, and additional trials are needed to determine this, taking into account GvHD severity, manifestations, and patient comorbidities.
Specific challenges when starting ECP tend to be limited to a small group of patients. Problems with low patient body weight have been addressed in recent years; however, patients with suboptimal vascular access remain poor candidates for ECP.16 The safety profile of ECP allows it to be utilized in most patients, including pediatric patients, however caution should be used in those who are at increased risk of infection.
Overall, ECP remains a safe and efficacious treatment for most patients with cGvHD, particularly when used after steroid failure. In the future, response rates could improve further as additional insights into the optimal combinations, treatment scheduling, and sequencing for ECP become available, especially with the development of newer novel agents.
This independent educational activity was supported by Mallinckrodt Pharmaceuticals. All content was developed independently. The funder was allowed no influence on the content of this activity.
Your opinion matters
After reading this article, I commit to reviewing the latest data on ECP for cGvHD, and apply my learnings to guide my management of patients with cGvHD in clinical practice.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
Which consideration most strongly guides your decision to escalate therapy in SR-aGvHD?



