All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional.
The gvhd Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the gvhd Hub cannot guarantee the accuracy of translated content. The gvhd and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The GvHD Hub is an independent medical education platform, sponsored by Medac and supported through grants from Sanofi and Therakos. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View GvHD content recommended for you
Acute and chronic GvHD: An overview
Do you know... Which of the following factors lowers the risk of developing GvHD?
Graft-versus-host disease (GvHD) is a systemic condition that occurs when immunocompetent T cells in the transplanted tissue (graft) attack the immunodeficient tissue of the recipient (host). GvHD is a common complication in patients undergoing allogeneic hematopoietic stem cell transplantation (allo-HSCT).1 Depending on the timing of the post-transplant occurrence of histocompatibility differences, GvHD is classified as1:
- Acute GvHD (aGvHD): usually occurs within 100 days
- Chronic GvHD (cGvHD): usually occurs after 100 days
- Persistent, recurrent, or late-onset aGvHD: clinical features of aGvHD but occurs after 100 days posttransplantation
- Overlap syndrome: occurs anytime post-transplantation and includes both acute and chronic GvHD symptoms
Etiology1
GvHD most commonly occurs following allo-HSCT. It can also manifest following the transfusion of un-irradiated blood and transplantation of solid organs (e.g., liver). Risk factors that increase the incidence of GvHD include unmatched donor transplants, human leukocyte antigen disparity, sex-mismatch between donor and recipient, and chemotherapy and radiation therapy causing total body irradiation. Cryopreservation of marrow, use of immunomodulators, and umbilical cord blood lower the incidence of GvHD.
Epidemiology
The occurrence of aGvHD in patients undergoing allo-HSCT from an human leukocyte antigen (HLA)-matched sibling ranges between 9% and 50%, with a higher occurrence in unmatched donors.2 Chronic GvHD occurs in 6–80% of patients and is one of the main causes of mortality following allo-HSCT (Figure 1).1, 3-9
Figure 1. Epidemiology of GvHD*
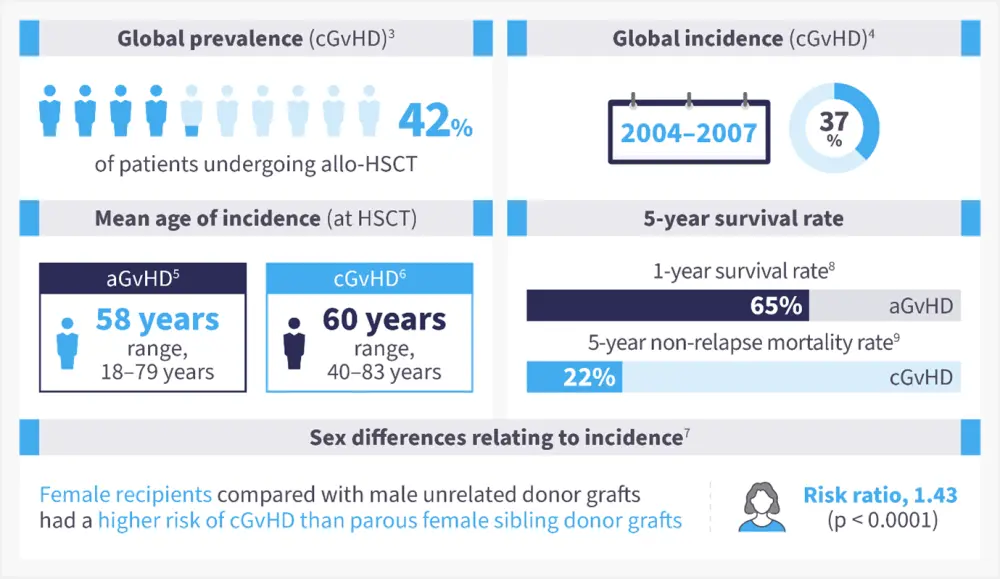
aGvHD, acute graft-versus-host disease; allo-HSCT, allogeneic hematopoietic stem cell transplant; cGvHD, chronic GvHD; US, United States.
*Adapted from Bachier, et al.3 ; Arai, et al.4 ; Akahoshi, et al.5; Bhatt, et al.6, Kumar, et al.7; Holtan, et al.8; DeFilipp, et al.9
Pathophysiology10
The pathophysiology of GvHD is divided into three phases (Figure 2):
- Conditioning-mediated tissue damage: In this phase, tissue damage occurs due to both the patient’s underlying condition and conditioning regimens used to treat the condition. This activates the host antigen-presenting cells.
- Donor T-cell activation or afferent phase: Donor T cells recognize allo-antigen either on host or donor antigen-presenting cells.
- Target cell apoptosis or efferent phase: Migration of cytotoxic T lymphocytes and natural killer cells to target organs, causing damage and multi-organ failure.
Figure 2. Pathophysiology of GvHD*
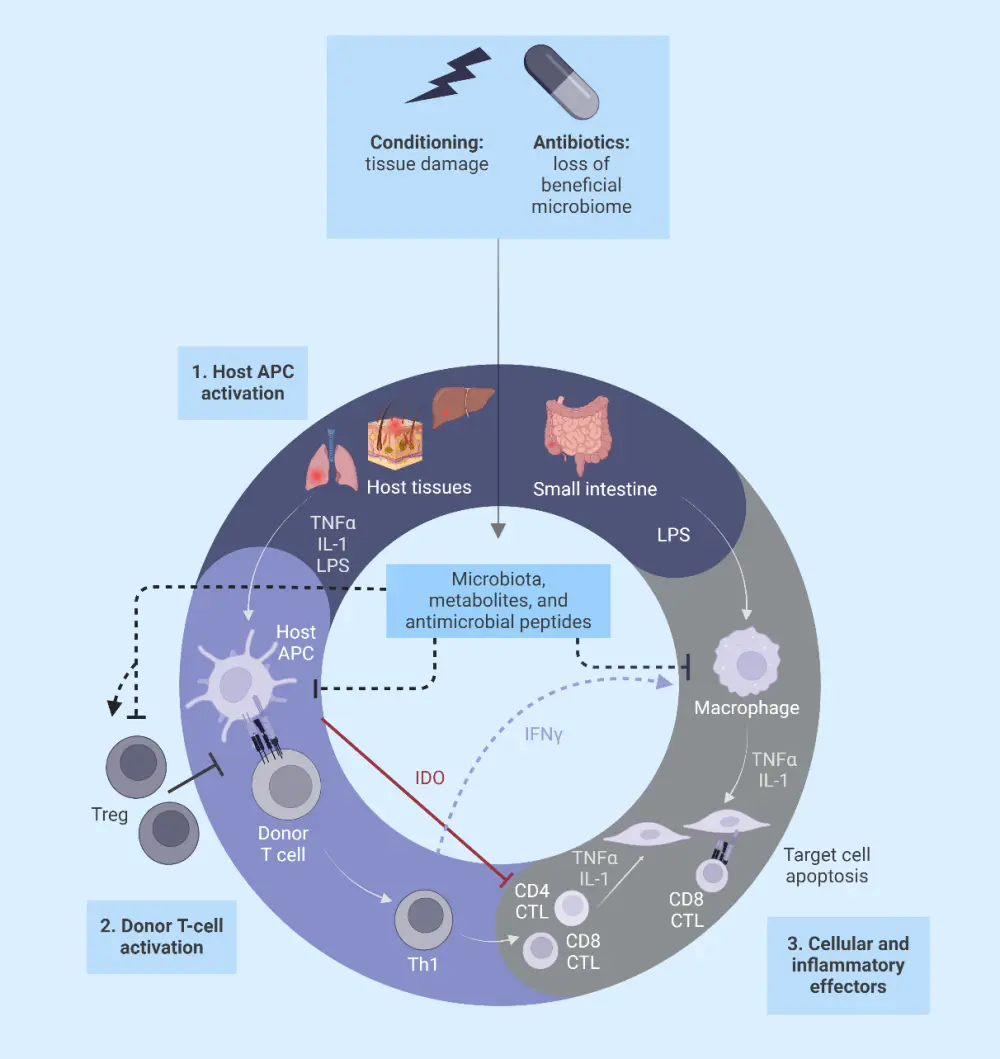
APC, antigen-presenting cell; CTL, cytotoxic T lymphocyte; GvHD, graft-versus-host disease; IDO, indoleamine 2, 3-dioxygenase; IFNγ, interferon gamma; LPS, lipopolysaccharide; TNFα, tumor necrosis factor alpha; Treg, regulatory T cell.
*Adapted from Ghimire, et al.10 Created with BioRender.com
Signs and symptoms
In aGvHD, signs and symptoms most commonly occur around the time of donor engraftment. The most common organs affected in aGvHD include the skin, gastrointestinal tract, and liver.2 In cGvHD, symptoms typically occur within the first year after HSCT, but can occur as late as 7 years post-HSCT. Figure 3 shows the symptoms of both aGvHD and cGvHD. Symptoms can be used to differentiate between cGvHD and aGvHD, although "overlap syndrome" can occur, where typical aGvHD symptoms are present in a patient diagnosed with cGvHD.11
Figure 3. Signs and symptoms of A aGvHD and B cGvHD*
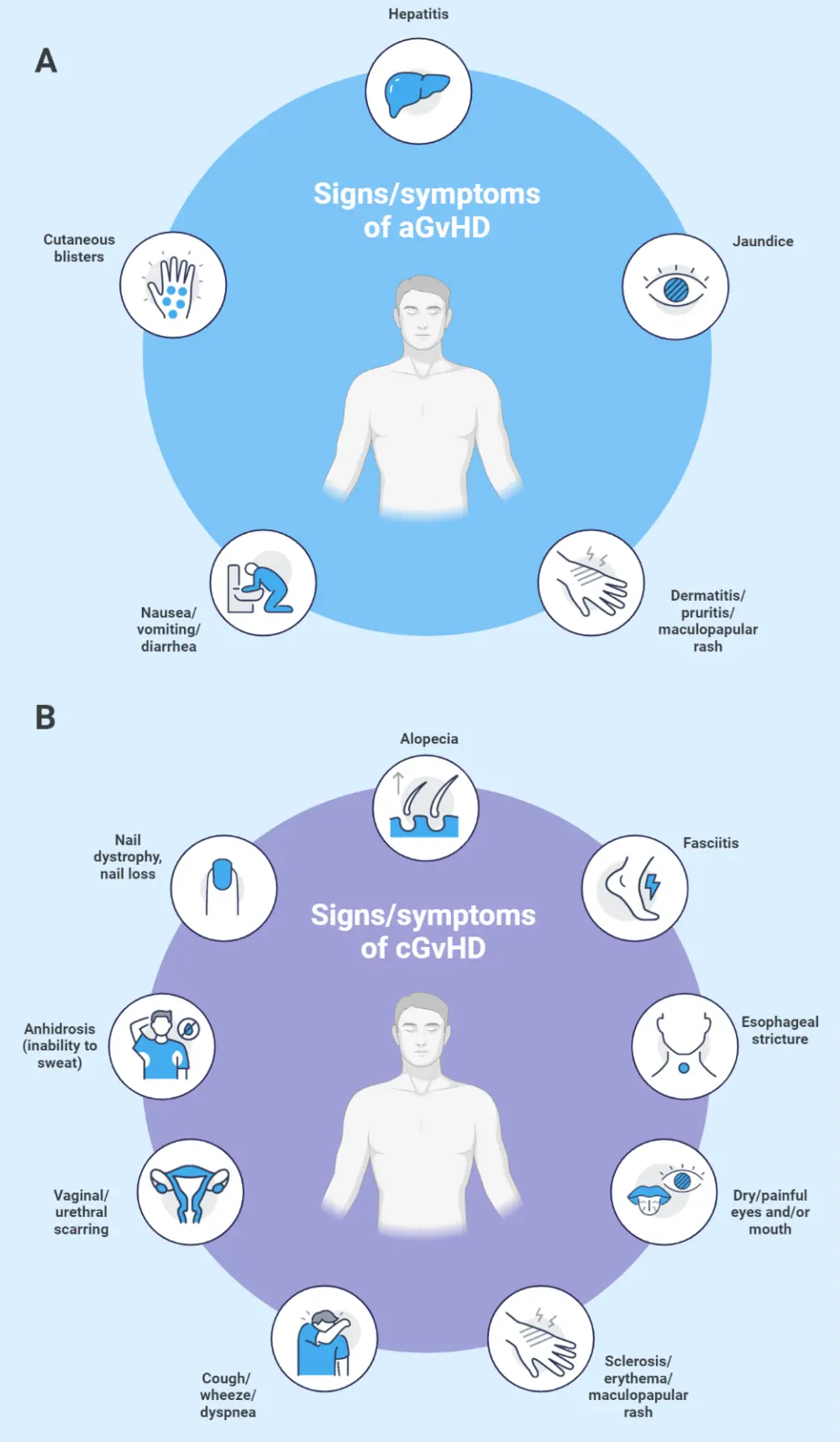
aGvHD, acute graft-versus-host disease; cGvHD, chronic GvHD.
*Adapted from Flowers and Martin.11; Jacobsohn and Vogelsang.12 Created with BioRender.com.
Diagnosis
Symptoms of aGvHD are used to make a diagnosis, and biopsy can be performed to confirm it.12 Liver biopsy in patients with GvHD may reveal irregular bile ducts or early cytotoxic lymphocyte attack on the bile ducts. Endoscopy often reveals edema, sloughing of the mucosa, and sometimes bleeding. Findings present in skin biopsies can vary, and can include dyskeratotic keratinocytes, basal cell necrosis, and depletion of Langerhans cells.12
Early recognition of cGvHD manifestation and timely assessment of the affected organs is necessary for the diagnosis and management of cGvHD. The most used tests to diagnose GvHD include biopsy, radiography, and other testing to exclude other diagnoses.11
GvHD grading is important for assessing patients’ response to prophylactic treatments, the impact on survival, and association with graft-versus-leukemia effect. There are several grading systems developed for aGvHD; the most commonly used is the International Bone Marrow Transplant Registry grading system, defining the severity of aGvHD as13:
- Grade 1: Stage 1 skin involvement alone with no liver or gastrointestinal involvement
- Grade 2: Stage 2 skin involvement; Stage 1 or 2 gut involvement
- Grade 3: Stage 3 involvement of any organ system
- Grade 4: Stage 4 involvement of any organ system
cGvHD grading is based on the National Institute for Health criteria as14:
- Mild: 1–2 organs involved; excluding lung
- Moderate: ≥3 organs involved; lung involvement is mild
- Severe: ≥3 organs involved; lung involvement is moderate
Guidance on diagnosis may vary between countries, please check the key guidelines section below.
Management
Prophylactic treatment in patients undergoing allo-HSCT is important for prevention of GvHD. The most commonly used prophylactic treatment is a combination of cyclosporine and methotrexate. In addition, antibacterial, antiviral, and antifungal prophylaxis are used to mitigate the risk of infections.1 Corticosteroids are the most commonly used treatment for GvHD, with the main focus of treatments being immunosuppression of donor T cells. Prednisone at a dose of 1 mg/kg should be used as a first-line treatment of newly diagnosed cGvHD.11
Treatments should focus on reducing the symptoms of GvHD while avoiding reducing the beneficial graft vs tumor response. The choice of treatment is based on the severity of symptoms and organ involvement. Figure 4 shows the criteria for treatment selection with corticosteroids.1
Figure 4. Treatment for GvHD*

GvHD, graft-versus-host disease.
*Adapted from Justiz Vaillant, et al.1
To prevent GvHD flares, gradual tapering of steroids is recommended. In patients receiving steroids for a longer period of time, octreotide can be given to reduce diarrhea. In addition, cyclosporine can be added to cGvHD treatment regimens to reduce steroid dosage.1
The GvHD Hub has reported on developments in the management of cGvHD. Currently, there are no standard second-line therapies for GvHD, and institutional advice should be followed depending on the location of the center; second-line therapies available for cGvHD are outlined in Figure 5.
Figure 5. Potential second-line therapies for cGvHD*
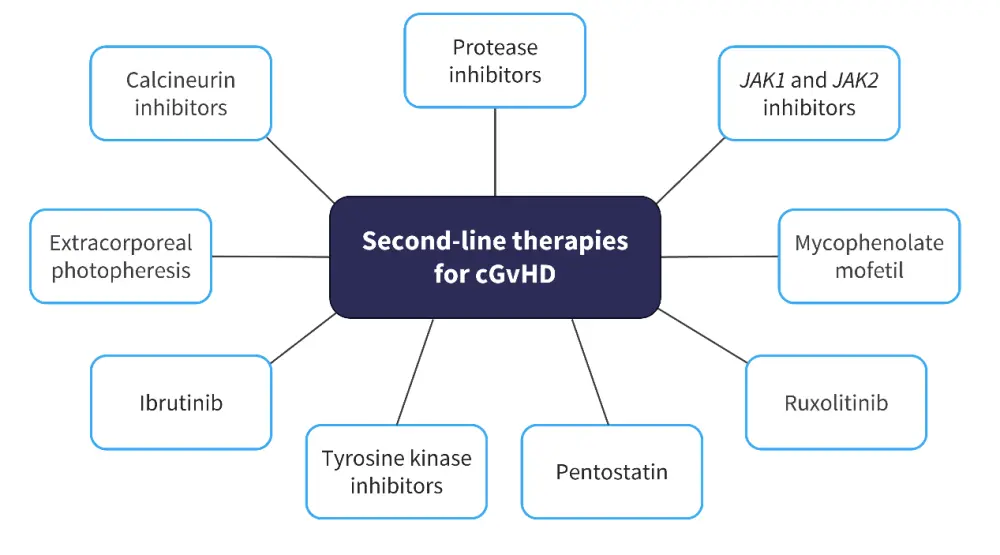
cGvHD, chronic graft-versus-host disease; JAK, Janus kinase.
*Data from GvHDHub.com.15
Guidance on management may vary between countries. Please see the section below on key guidelines for further information.
Key guidelines and organizations
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
Which consideration most strongly guides your decision to escalate therapy in SR-aGvHD?

