All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional.
The gvhd Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the gvhd Hub cannot guarantee the accuracy of translated content. The gvhd and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The GvHD Hub is an independent medical education platform, sponsored by Medac and supported through grants from Sanofi and Therakos. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View GvHD content recommended for you
Editorial theme | Consensus statements for pediatric GvHD: Recommendations from the EBMT Transplant Complications Working Party
Graft-versus-host disease (GvHD) remains a considerable contributor to mortality and morbidity in patients receiving allogeneic hematopoietic stem-cell transplantation (allo-HSCT). Recommendations on the management of GvHD were consolidated and published in 2014 by a European Society of Blood and Marrow Transplantation (EBMT)/European Leukaemia Net (ELN) working group. There have since been advances in studies evaluating different elements of GvHD care, and updated guidelines for the prevention and management of GvHD following allo-HSCT in patients with hematologic malignancies were recently published in Lancet Hematology.1
Defining routine guidelines for pediatric patients with GvHD has proven challenging, but the updated recommendations cover some specific viewpoints in this difficult setting. Management strategies of pediatric patients with GvHD share some commonalities with the adult population, but with exceptions and deviations.
Over the coming months, the GvHD Hub will be exploring different avenues of pediatric GvHD including genetic predisposition, diagnosis, emerging therapies, and refining stem cell transplantation in this population. As an introduction to this editorial theme, the GvHD Hub are pleased to present a summary of the consensus statements around the management of GvHD following allo-HSCT, concluding with those specific to pediatric patients and where they differ.
Background
Five senior hematologists formed a task force challenged with compiling guidelines for the prevention and treatment of GvHD in patients receiving transplantations from a matched sibling or matched unrelated donor for the treatment of hematologic malignancies. In total, 38 statements reached consensus (95% or 100% of a 20-person expert panel agreed) and the following definitions and criteria were used throughout for uniformity:
- Pediatric: ≤17 years of age
- Acute GvHD: Mount Sinai Acute GvHD International Consortium criteria
- Chronic GvHD: National Institutes of Health (NIH) 2014 criteria
- Steroid-refractoriness/ -resistance/ -dependence: EBMT−NIH−Center for International Blood and Marrow Transplant Research (CIBMTR) Task Force position statement
Consensus statements
The guidelines were classified into four main categories and are summarized below (Figures 1–4). Recommendations in bold represent those applicable to, or amended for, the pediatric population, and are expanded on in Figure 5.
GvHD prophylaxis
Consensus statements for the GvHD prevention and prophylaxis are summarized in Figure 1.
Figure 1. Summary of consensus guidelines around prophylaxis approaches to patients undergoing allo-HSCT*
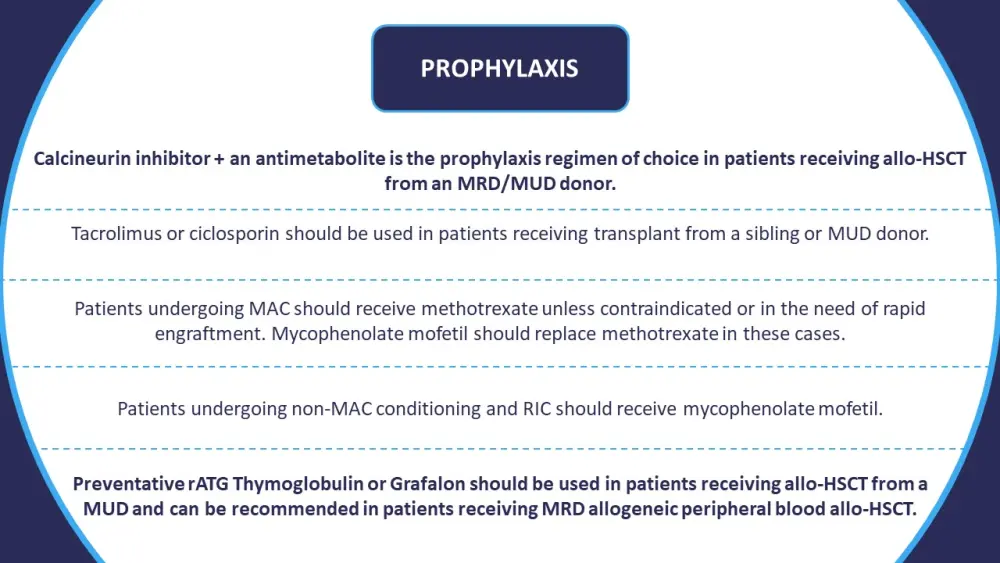
Allo-HSCT, allogeneic hematopoietic stem cell transplantation; MAC, myeloablative conditioning; MRD, matched related donor; MUD, matched unrelated donor; RIC, reduced-intensity conditioning; rATG, rabbit anti-thymocyte globulin.
*Information from Penack, et al.1
Prophylaxis management
Despite a lack of comparative studies evaluating the management of therapies during prophylaxis in patients undergoing allo-HSCT, consensus was reached for several recommendations (Figure 2)
Figure 2. Summary of consensus guidelines on treatment management during GvHD prophylaxis*

GvHD, graft-versus-host disease; IV, intravenous; MAC, myeloablative conditioning; MRD, matched related donor; MUD, matched unrelated donor; RIC, reduced-intensity conditioning; rATG, rabbit anti-thymocyte globulin.
*Information from Penack, et al.1
† Ciclosporin concentration samples should not be obtained from the lines used for infusion.
‡15–30 mg/kg rATG Grafalon has demonstrated reasonable efficacy in non-randomized studies.
aGvHD
Treatment approaches to aGvHD largely aim to reduce unnecessary systemic treatment and are limited to Grade ≥2 disease. There is no single approach to second-line therapy in the aGvHD setting (Figure 3).
Figure 3. Summary of consensus guidelines on the treatment of aGvHD*

aGvHD; acute graft-versus-host disease; CR, complete response; FDA, U.S. Food and Drug Administration; GI, gastrointestinal; rATG, rabbit anti-thymocyte globulin.
*Information from Penack, et al.1
cGvHD
Steroids remain the initial treatment of choice for patients with cGvHD. The benefit of front-line combination regimens has not been evidenced in patients with moderate cGvHD; however, limiting steroid use with the addition of alternative immunosuppressants has been recommended in the case of severe cGvHD. Like aGvHD, second-line therapy for cGvHD has not been standardized (Figure 4).
Figure 4. Summary of consensus guidelines on the treatment of cGvHD*

cGvHD, chronic graft-versus-host disease; CI, calcineurin inhibitor; FAM, fluticasone, azithromycin, and montelukast; FDA, U.S. Food and Drug Administration; MRD, minimal residual disease.
*Information from Penack, et al. and U.S. Food and Drug Administration.1,2
†as defined by NIH classifications
‡where the initial dose is <1 mg/kg
Pediatric GvHD
Figure 5 demonstrates the specific recommendations that are applicable to, or have been revised for, pediatric application.
Figure 5. Summary of consensus statements specific to pediatric patients with GvHD*
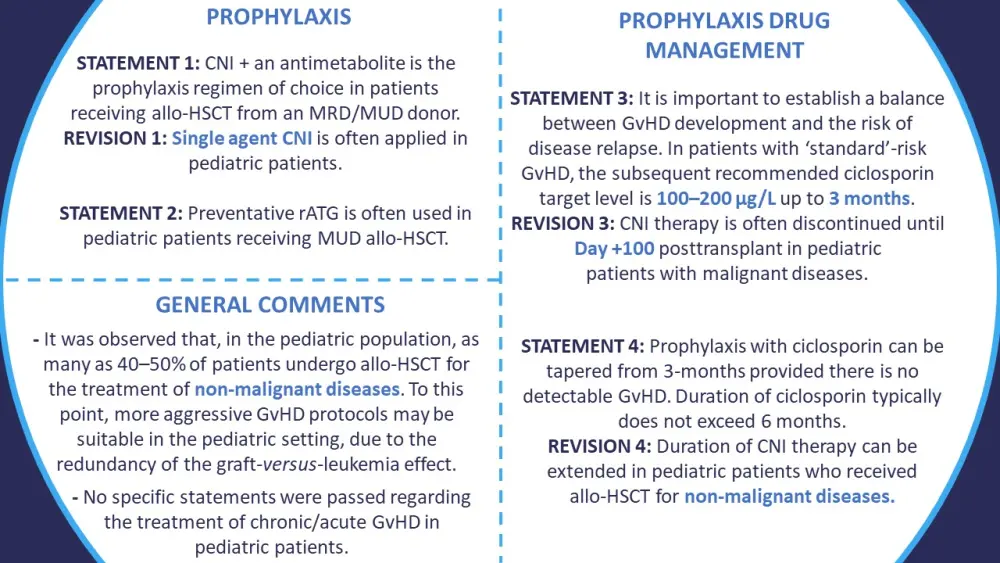
Allo-HSCT, allogeneic stem cell transplant; CNI, calcineurin inhibitors; GvHD, graft-versus-host disease; MRD, matched related donor; MUD, matched unrelated donor; rATG, rabbit anti-thymocyte globulin.
*Information from Penack, et al.1
Conclusion
Efforts have been made to identify common guidelines for the prevention, management, and treatment of GvHD. The recently published summary goes someway to address these measures in pediatric patients; however, specific regulations remain limited. Through this editorial theme, the GvHD Hub aims to provide further insights in the pediatric GvHD setting.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
Which consideration most strongly guides your decision to escalate therapy in SR-aGvHD?

