All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional.
The gvhd Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the gvhd Hub cannot guarantee the accuracy of translated content. The gvhd and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The GvHD Hub is an independent medical education platform, sponsored by Medac and supported through grants from Sanofi and Therakos. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View GvHD content recommended for you
Developments in the management of cGvHD
Chronic graft-versus-host disease (cGvHD) occurs in 30–50% of patients receiving allogeneic hematopoietic stem cell transplantation with heterogeneous clinical manifestations.1 In their recent review, Holtzman and Pavletic summarize developments in the field of cGvHD that have occurred since the National Institutes of Health (NIH) consensus conference in 2014.
Figure 1 details a diagnostic, classification, and treatment algorithm laid out by Holtzman and Pavletic, which demonstrates the complexity of cGvHD management.
Figure 1. A diagnostic, classification, and treatment algorithm for cGvHD*
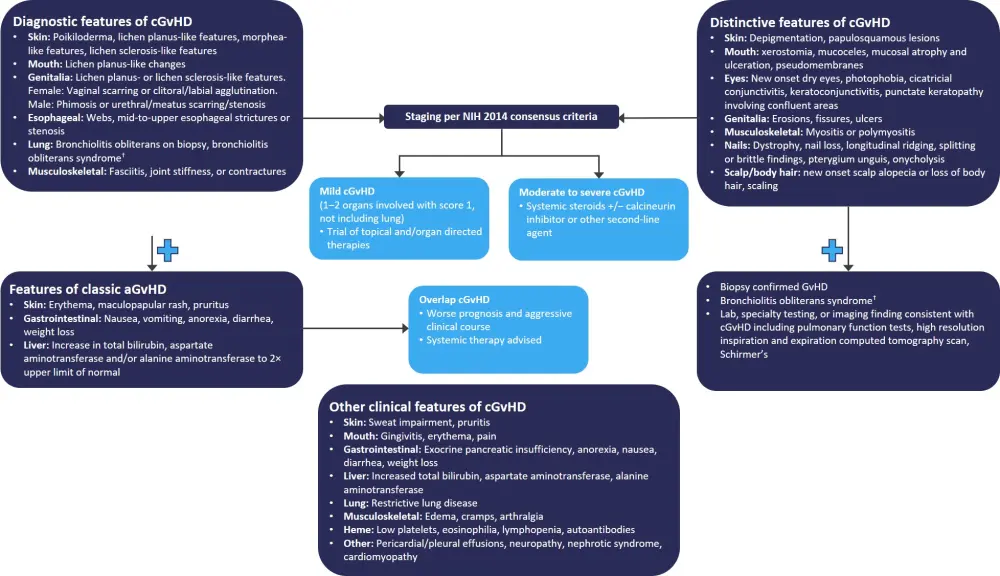
aGvHD, acute graft-versus-host disease; cGvHD, chronic graft-versus-host disease; NIH, National Institutes of Health.
*Adapted from Holtzman and Pavletic.1
†Bronchiolitis obliterans syndrome is only diagnostic for lung cGvHD if there is a distinctive sign or symptom in another organ.
Front-line therapy
There have only been a few studies attempting to optimize front-line GvHD treatment since 2014. An important study comparing prednisone/sirolimus vs prednisone/sirolimus/calcineurin inhibitor demonstrated that the two-drug induction regimen yielded similar clinical benefits as the three-drug regimen, but with a better toxicity profile. Another study assessing extracorporeal photopheresis (ECP) alongside standard of care, compared to standard of care, found no benefit in terms of response, quality of life, or toxicity.
Second-line therapy
With >50% of patients becoming steroid-dependent or -refractory, second-line therapies are a vital part of the cGvHD treatment pathway. Steroid-refractory disease is defined as: disease that has progressed despite treatment with prednisone (1 mg/kg/day) for 2 weeks, disease that is stable despite treatment with prednisone (0.5 mg/kg/day) for 4–8 weeks, or an inability to taper prednisone treatment below 0.5 mg/kg/day. Steroid-dependent disease definitions vary in clinical practice, though one suggested definition is: disease that flares when prednisone is tapered below 0.25 mg/kg/day on more than one taper attempt (≥8 weeks between).
Progression within 4 weeks or no response to front-line therapy within 8–12 weeks signals the need for second-line therapies. There are no standardized protocols for the selection of second-line treatments.
Studies of agents that may be used for refractory cGvHD, including full clinical study reports and smaller clinical studies, since 2014 are detailed in Table 1.
Table 1. Key published studies for steroid-refractory cGvHD*
|
AE, adverse event; AF, atrial fibrillation; ALT, alanine aminotransferase; CHF, congestive heart failure; CMV, cytomegalovirus; CNI, calcineurin inhibitor; CPK, creatine phosphokinase; CR, complete response; DVT/PE, deep-vein thrombosis/pulmonary embolism; ECP, extracorporeal photopheresis; FFS, failure-free survival; GBS, Guillain–Barre syndrome; GI, gastrointestinal; HF, heart failure; IV, intravenous; LD-IL-2, low-dose interleukin-2; N/A, not available; ORR, overall response rate; OS, overall survival; P, prospective; R, retrospective; SBO, small bowel obstruction; SVC, superior vena cava; TB, tuberculosis; y, year(s). |
||||||
|
Agent |
Studies |
N |
Severe cGvHD, % |
Outcomes, % |
Important AEs |
Comments |
|---|---|---|---|---|---|---|
|
Ibrutinib |
1 P |
42 |
N/A |
ORR, 67 |
Pneumonia, fatigue, diarrhea, infections |
Caution advised in cases of AF, anticoagulation and bleeding history, invasive fungal infections, taking strong CYP3A4 |
|
Ruxolitinib |
6 R and 2 P |
19–165 |
33–86 |
ORR, 48–100 |
Infection, CMV reactivation, cytopenia, hepatic impairment, heme toxicity, lung TB |
Close monitoring for: infection, cytopenias, hyperlipidemia |
|
ECP |
2 P, plus 1 other |
49–88 |
43–59 |
ORR, 43.5–80 |
Infection |
Important quality of life implications |
|
ECP + LD-IL-2 |
1 P |
25 |
24 |
ORR, 62 |
Dehydration, hypotension, syncope, anemia |
IL-2 therapy associated with thrombotic microangiopathy, concurrent therapy with CNIs or sirolimus is discouraged |
|
LD-IL-2 |
1 P |
35 |
20 |
ORR, 61 |
Flu-like symptoms, fatigue, DVT/PE, myalgia/arthralgia, hepatitis, psychiatric disturbances |
|
|
Rituximab vs imatinib |
1 P |
75 |
100 sclerotic |
ORR, 27 vs 26 |
Imatinib: infection Rituximab: infection, infusion reaction, neutropenia |
Rituximab associated with infusion reactions and prolonged hypogammaglobulinemia requiring IV immunoglobulin supplementation |
|
Rituximab + nilotinib |
1 P |
29 |
76 |
ORR, 71 |
Infection, encephalitis, osteomyelitis, dyspnea, avascular necrosis, anemia, leukopenia, prolonged QTc, nausea, pain, fatigue, pancreatitis, GBS-like neurologic syndrome |
|
|
Nilotinib |
1 P |
21 |
100 |
ORR, 22.2–55.6† |
ALT increase, lipase/amylase increase, infection (meningitis), anemia |
|
|
Imatinib |
1 P |
20 |
100 sclerotic |
ORR, 36 |
Edema, myalgia, muscle cramps, pleural effusions, hypophosphatemia, nausea, diarrhea, fatigue, tinnitus |
Caution in patients with fluid retention, history of pleural effusions/HF. |
|
Pomalidomide |
2 P |
13–34 |
85–94 |
ORR, 54–67 |
Lymphopenia, infection, fatigue, neuropathy, myalgias, tremors, fatigue/anxiety, DVT/PE |
Requires concurrent thromboprophylaxis (aspirin and/or other anticoagulation) |
|
Ixazomib |
1 P |
50 |
84 |
ORR, 40 |
Thrombocytopenia, diarrhea, fatigue, infection, respiratory failure |
Associated with rash, GI toxicity, neuropathy, and rarely, thrombotic microangiopathy. |
|
Belumosudil |
1 P |
132 |
67 |
ORR, 76 |
Pneumonia, hypertension, hyperglycemia, increased liver function test |
Efficacious in patients having previously received ibrutinib and/or ruxolitinib |
|
Tocilizumab |
1 R |
11 |
100 |
ORR, 70 |
Infection |
|
|
Ofatumumab |
1 P |
12 |
42 |
ORR, 80 |
Fatigue, infusion reactions, lung, and upper respiratory infection |
No dose-limiting toxicities |
|
Abatacept |
1 P |
17 |
59 |
ORR, 44 |
Pulmonary infections |
No dose-limiting toxicities |
|
Sonidegib |
1 P |
17 |
59 |
ORR, 47 |
Arthralgia, abdominal pain, myalgia, back pain, headache, hypercalcemia, anemia, cardiac arrest, CHF hypertension, diarrhea, SBO, SVC syndrome |
Dose-limiting toxicities: increase in CPK |
Organ-specific challenges
Sclerotic skin
Cutaneous sclerosis affects 10–20% of patients and is one of the most challenging cGvHD manifestations. It can lead to a poor quality of life (QoL) due to skin stiffness leading to limited range of motion, movement restrictions, and effects on other organs. Although the pathophysiology behind sclerotic skin is yet to be elucidated, there has been interest in certain agents due to their potential antifibrotic effects. These include ruxolitinib, imatinib, low-dose IL-2, pomalidomide, belumosudil, ibrutinib, and rituximab.
Pulmonary
Affecting 5–12% of HSCT patients, bronchiolitis obliterans syndrome is another challenging cGvHD manifestation that is associated with decreased QoL, high morbidity, and high mortality. Although a 2016 study established FAM (fluticasone, azithromycin, and montelukast) as the standard front-line bronchiolitis obliterans syndrome treatment, the study findings have not been reproduced. ECP has shown promise in a small retrospective report, with a 100% response rate in six patients who had received prior FAM treatment. An alternative option seems to be lung transplantation, which has shown 100% survival at 19.5 months of follow up and 37% survival at 5 years.
Myofascial
This manifestation of cGvHD has a significant effect on patient QoL and mobility. Myofascial cGvHD is difficult to quantify, but a refined algorithm of the NIH response criteria has been proposed recently to improve response measurements. In retrospective analyses, the best responses were found to be following treatment with rituximab, imatinib, ECP, and bortezomib.
Topical therapies
In cases of mild cGvHD, topical organ-specific therapies are the main option and can enable patients who experience toxicity from systemic treatment to reduce those and benefit from topical treatments instead. Holtzman and Pavletic highlight the importance of a multidisciplinary approach to cGvHD management. Figure 2 details the management recommendations suggested for cGvHD that incorporate interdisciplinary, ancillary, and supportive care.
Skin
Topical steroids are the main treatment for skin cGvHD, provided that the disease is present in <50% of the skin and body surface area.
Oral
The priority treatment for oral cGvHD are high-potency topical corticosteroid rinses. Other symptoms may include a dry mouth, which can be treated with sialogogues and salivary stimulants. Dexamethasone has been shown to reduce the oral sensitivity score more than tacrolimus (58% vs 21%; p = 0.05) with similar safety profiles. Platelet gel has also been found to show promise as an option for patients with oral cGvHD (complete response, 70%; overall response, 27.3–43.2%).
Ocular
An estimated 35–51% of patients with cGvHD experience ocular involvement, with significantly impacted QoL. Topical treatments currently include artificial tears, eye drops, ointments, immunosuppressive eye drops, warm compresses and lid hygiene, punctal plugs, and scleral lenses. There have been promising studies into topical steroids vs tacrolimus, platelet-derived eye drops, autologous serum eye drops, and scleral lenses. There have also been smaller studies of combined JAK/spleen tyrosine kinase (SYK) inhibitor ophthalmic eye solution, a mucin secretagogue eye drop (3% diquafosol ophthalmic solution), and a lymphocyte function-associated antigen-1 antagonist (lifitegrast).
Genital
Topical steroids or calcineurin inhibitors are the main treatment options for patients with vulvovaginal or penile cGvHD. Estrogen replacement may also be used if vulvovaginal cGvHD is associated with low estrogen levels.
Figure 2. Recommendations for interdisciplinary, system-based ancillary, and supportive care in cGvHD*
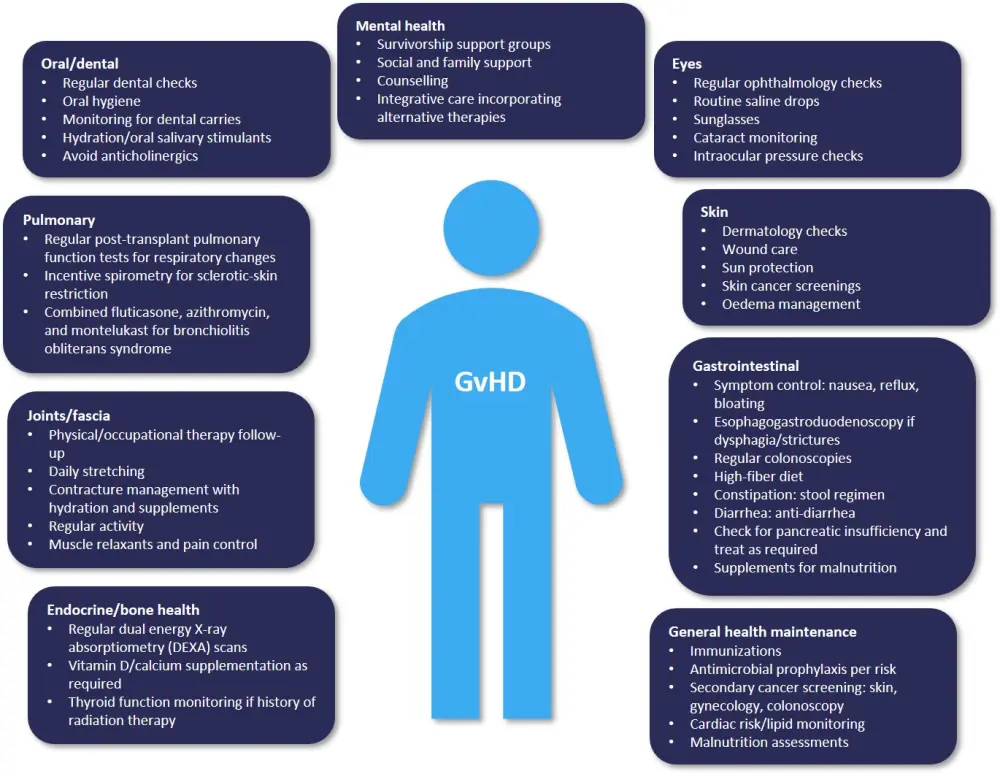
cGvHD, chronic graft-versus-host disease.
*Adapted from Holtzman and Pavletic.1
Future directions
Despite the many recent advances in the treatment of cGvHD, there is much more work to be done. There are several active studies ongoing (Table 2), but further research is needed in areas such as new therapies, combinatorial treatments, organ-specific approaches, steroid-free or steroid-deprived regimens, and treatment algorithms for personalized treatments.
Table 2. Active clinical trials for cGvHD*†
|
BOS, bronchiolitis obliterans syndrome; BTK, Bruton’s tyrosine kinase; cGvHD, chronic graft-versus-host disease; CSF-1, colony-stimulating factor 1; ECP, extracorporeal photopheresis; FGFR, fibroblast growth factor receptor; IL-2, interleukin-2; ITK, interleukin-2-inducible kinase; n/a, not available; JAK, Janus kinase; LD IL-2, low-dose interleukin-2; mTOR, mechanistic target of rapamycin; MSC, mesenchymal stromal cell; NRTK, non-receptor tyrosine kinase; PDGFR, platelet-derived growth factor receptor; ROCK2, rho-associated coiled-coil-containing protein kinase-2; RTK, receptor tyrosine kinase; SR, steroid-refractory; Treg, regulatory T cell; VEGF, vascular endothelial growth factor. |
||||||
|
Agent |
Target |
Phase |
Disease status |
Treatment |
Clinicaltrials.gov identifier |
Study site country |
|---|---|---|---|---|---|---|
|
Acalabrutinib |
BTK/ITK inhibitor |
II |
SR-GVHD |
Second-line |
NCT04198922 |
USA |
|
Anlotinib |
anti-VEGF, -FGFR, -PDGFR |
II |
SR-GVHD |
Second-line |
NCT04232397 |
China |
|
AMG 592 |
IL-2 cytokine mutein |
I/II |
SR-GVHD |
Second-line |
NCT03422627 |
USA, Belgium, France, Japan |
|
Arsenic trioxide |
N/A |
II |
cGVHD |
First-line |
NCT02966301 |
France |
|
Axatilimab (SNDX-6352) |
CSF-1R antibody |
I/II |
Refractory cGVHD |
Second-line |
NCT03604692 |
USA |
|
Axatilimab (SNDX-6352) |
CSF-1R antibody |
II |
Refractory cGVHD |
Second-line |
NCT04710576 |
USA |
|
Baricitinib |
JAK1/2 |
I/II |
SR-GVHD |
Second-line |
NCT02759731 |
USA |
|
Donor Tregs |
Adoptive immunotherapy |
I/II |
SR-cGvHD |
Second-line |
NCT02385019 |
Portugal |
|
Donor Tregs |
Adoptive immunotherapy |
I |
cGVHD refractory to steroids and ruxolitinib |
Second-line |
NCT03683498 |
Spain |
|
Donor-derived purified Tregs (Treg DLI) |
Adoptive immunotherapy |
I |
SR-cGVHD |
Second-line |
NCT02749084 |
Italy |
|
ECP + LD-IL2 |
Cytokine |
II |
SR-cGVHD |
Second-line |
NCT03007238 |
USA |
|
Everolimus and prednisone |
mTOR inhibitor |
IIa |
cGvHD |
First-line |
NCT01862965 |
Germany |
|
Glasdegib |
Hedgehog inhibitor |
I/II |
Refractory cGVHD with sclerosis and/or fasciitis |
Second-line |
NCT04111497 |
USA |
|
Glasdegib |
Hedgehog inhibitor |
I/II |
Sclerotic refractory cGVHD |
Second-line |
NCT03415867 |
Spain |
|
Ibrutinib |
BTK/ITK inhibitor |
III |
SR-cGVHD |
Second-line |
NCT03474679 |
Japan |
|
Ibrutinib + corticosteroids |
BTK/ITK inhibitor |
III |
cGVHD |
First-line |
NCT02959944 |
USA, Australia, Austria, Canada, China, Croatia, France, Germany, Hungary, Italy, Japan, Republic of Korea, Singapore, Spain, Taiwan |
|
Ixazomib |
Proteasome inhibitor |
II |
cGvHD |
Prophylaxis/ |
NCT03082677 |
USA |
|
Ixazomib |
Proteasome inhibitor |
Ib/II |
cGVHD |
Prophylaxis/ |
NCT03225417 |
Spain |
|
Itacitinib + corticosteroids |
JAK1 inhibitor |
II/III |
cGVHD |
First-line |
NCT03584516 |
USA, Austria, Belgium, Canada, France, Germany, Greece, Israel, Italy, Poland, Spain, Switzerland, UK |
|
KD025 |
ROCK2 inhibitor |
II |
cGVHD |
Second-line |
NCT02841995, NCT03640481 |
USA |
|
Leflunomide |
Pyrimidine synthesis inhibitor |
I |
SR-cGVHD |
Second-line |
NCT04212416 |
USA |
|
MSCs |
Adoptive immunotherapy |
II |
cGvHD |
First-line |
NCT04692376 |
China |
|
Obinutuzumab |
anti-CD20 monoclonal antibody |
II |
cGvHD |
Prophylaxis/ |
NCT02867384 |
USA |
|
Ofatumumab |
anti-CD20 monoclonal antibody |
I/II |
cGVHD |
First-line |
NCT01680965 |
USA |
|
Rituximab + ibrutinib |
anti-CD20 monoclonal antibody; BTK/ITK inhibitor |
II |
cGvHD |
First-line |
NCT04235036 |
USA |
|
Rituximab + ibrutinib |
anti-CD20 monoclonal antibody; BTK/ITK inhibitor monoclonal antibody; |
I/II |
SR-cGVHD |
Second-line |
NCT03689894 |
USA |
|
Ruxolitinib |
JAK1/2 |
II |
Sclerotic cGVHD |
Second-line |
NCT03616184 |
USA |
|
Ruxolitinib |
JAK1/2 |
III |
SR-cGVHD |
Second-line |
NCT03112603 |
USA, Australia, Austria, Belgium, Bulgaria, Canada, Czechia, Denmark, France, Germany, Greece, Hungary, India, Israel, Italy, Japan, Jordan, Republic of Korea, Netherlands, Norway, Poland, Portugal, Romania, Russia, Saudi Arabia, Spain, Sweden, Switzerland, Turkey, UK |
|
SCM-CGH/clonal MSCs |
Adoptive immunotherapy |
II |
SR-cGvHD |
Second-line |
NCT04189432 |
Republic of Korea |
|
Treg-enriched infusion + IL-2 |
Adoptive immunotherapy + cytokine |
I |
SR-cGvHD |
Second-line |
NCT01937468 |
USA |
|
Organ specific and topical agents |
||||||
|
Amniotic fluid eye drops (AFED) |
N/A |
I/II |
Ocular cGVHD |
Topical/organ specific |
NCT03298815 |
USA |
|
Alvelestat |
Neutrophil elastase inhibitor |
I/II |
BOS/lung cGVHD |
Organ specific |
NCT02669251 |
USA |
|
Itacitinib |
JAK1 inhibitor |
I |
BOS/lung cGVHD |
Organ specific |
NCT04239989 |
USA |
|
Nintedanib |
NRTK and RTK inhibitor |
II |
BOS/lung cGVHD |
Organ specific |
NCT03805477 |
Austria, Germany, Switzerland |
|
Pirfenidone |
Antifibrotic |
I |
BOS/lung cGVHD |
Organ specific |
NCT03315741 |
USA |
|
1% progesterone topical gel |
Progesterone |
II |
Ocular cGVHD |
Topical/organ specific |
NCT03990051 |
USA |
|
Ruxolitinib |
JAK1/2 |
II |
BOS/lung cGVHD |
Organ specific |
NCT03674047 |
USA |
|
Ruxolitinib 1.5% topical cream |
JAK1/2 |
II |
Cutaneous cGVHD |
Topical/organ specific |
NCT03395340 |
USA |
|
Umbilical mesenchymal stem cell-derived exosomes |
Adoptive immunotherapy |
I/II |
Ocular cGVHD |
Topical/organ specific |
NCT04213248 |
China |
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
Which consideration most strongly guides your decision to escalate therapy in SR-aGvHD?

