All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional.
The gvhd Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the gvhd Hub cannot guarantee the accuracy of translated content. The gvhd and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The GvHD Hub is an independent medical education platform, sponsored by Medac and supported through grants from Sanofi and Therakos. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View GvHD content recommended for you
Preventing GvHD with posttransplant cyclophosphamide combined with cyclosporin A: Results from HOVON-96
Allogeneic hematopoietic stem cell transplantation (allo-HSCT) is potentially curative for a range of hematologic malignancies; however, graft-versus-host disease (GvHD) remains a significant and important complication associated with high mortality and reduced quality of life.1
The most widely used regimen for GvHD prophylaxis after myeloablative transplant consists of cyclosporin A (CsA) and methotrexate, with European guidelines recommending the addition of antithymocyte globulin. Standard prophylaxis after non-myeloablative transplant differs, consisting of CsA in combination with mycophenolate mofetil. More recently, posttransplant cyclophosphamide (PTCy) alone or combined with CsA has shown promising GvHD prophylactic potential.1
The HOVON-96 trial (NL2128) investigated prophylactic treatment with PTCy in combination with a short course of CsA (PTCy/CsA), compared with conventional immunosuppression with CsA and mycophenolic acid (CsA/MPA) after non-myeloablative allo-HSCT. The results, which have recently been published by Broers et al.1 in Blood Advances, are summarized below.
Study design
- The HOVON-96 trial was a prospective, randomized, phase III trial investigating GvHD prophylaxis with PTCy/CsA versus CsA/MPA.
- Patients diagnosed with a high-risk hematologic malignancy who were due to undergo allo‑HSCT, were aged 18–70 years, and who had a World Health Organization performance status between 0 and 2 were eligible for the study.
- Patients also had to have a matched related donor or matched unrelated donor with at least 8 out of 8 human leukocyte antigen allele matches.
- Severe renal dysfunction, active infection, progressive or refractory disease, and inclusion of antithymocyte globulin in the conditioning regimen were criteria for exclusion.
- The conditioning regimen was at the discretion of the treating physician for participants receiving CsA/MPA. For those receiving PTCy/CsA, the conditioning regimen was specified and modified from the non-myeloablative Seattle protocol.
- Patients were randomly assigned to receive CsA/MPA or PTCy/CsA in a 1:2 ratio. The dosing regimen is shown in Figure 1.
- Primary endpoint: the proportion of patients with non-severe GvHD within 180 days posttransplant (PG180).
- Non-severe GvHD was defined as acute GvHD (aGvHD) Grade I, aGvHD Grade II without gut involvement, or chronic GvHD (cGvHD) not requiring treatment within 180 days after randomization.
- Secondary endpoints: time from transplantation to aGvHD Grade ≥I, ≥II, ≥III, and ≥IV, time to limited/extensive and extensive cGvHD, incidence of relapse/progression, non-relapse mortality, progression-free survival, overall survival, adverse events, and GvHD-free, relapse free survival (GRFS).
Figure 1. Dosing regimen for PTCy/CsA and CsA/MPA*
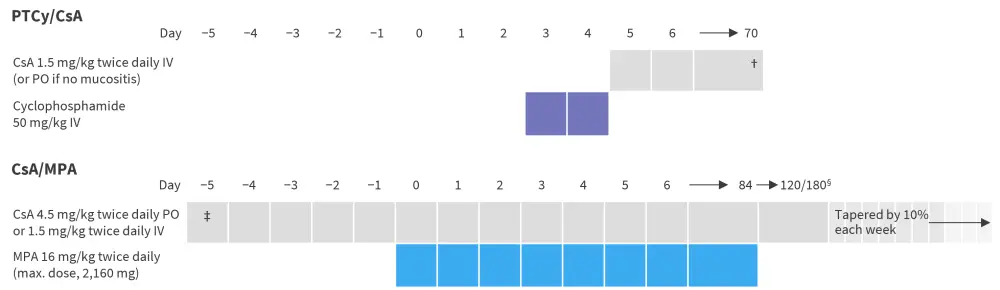
CsA, cyclosporine A; IV, intravenously; MPA, mycophenolic acid; PO, orally; PTCy, posttransplant cyclophosphamide.
*Data from Broers, et al.1
†CsA stopped without tapering at Day 70 in patients with GvHD.
‡CsA started between 3 and 5 days prior to transplant.
§CsA tapering started at Day 120 for patients without GvHD and from Day 180 for patients with GvHD.
Baseline characteristics
There were no significant differences in baseline characteristics between the cohorts, as shown in Table 1. Overall, there were more males than females, and most patients had acute myeloid leukemia or non-Hodgkin lymphoma.
Table 1. Baseline patient characteristics*
|
ALL, acute lymphoblastic leukemia; AML, acute myeloid leukemia; CLL, chronic lymphocytic leukemia, CML, chronic myeloid leukemia; CMV, cytomegalovirus; CsA/MPA, cyclosporine A and mycophenolic acid; MDS, myelodysplastic syndrome; MM, multiple myeloma; MPD, myeloproliferative disease; MRD, matched related donor; MUD, matched unrelated donor; PTCy/CsA posttransplant cyclophosphamide and cyclosporine A. |
||
|
Characteristic, % (unless otherwise stated) |
CsA/MPA |
PTCy/CsA |
|---|---|---|
|
Median age (range), years |
58 (26–70) |
57 (20–70) |
|
Sex, male/female |
63/37 |
67/33 |
|
Diagnosis |
|
|
|
AML |
27 |
30 |
|
ALL |
13 |
10 |
|
MDS |
15 |
8 |
|
CML |
2 |
5 |
|
CLL |
4 |
6 |
|
NHL |
19 |
21 |
|
HL |
4 |
3 |
|
MPD |
2 |
3 |
|
MM |
4 |
7 |
|
Other |
10 |
6 |
|
Donor type |
|
|
|
MRD |
33 |
30 |
|
MUD |
67 |
70 |
|
Female donor/male recipient pairs |
19 |
17 |
|
CMV status |
|
|
|
Recipient positive, donor positive |
31 |
39 |
|
Recipient negative, donor negative |
40 |
34 |
|
Recipient positive, donor negative |
15 |
18 |
|
Recipient negative, donor positive |
13 |
8 |
|
Conditioning regimen |
|
|
|
Myeloablative |
4 |
— |
|
Reduced intensity |
2 |
1 |
|
Non-myeloablative |
94 |
99 |
|
Source of stem cells |
|
|
|
Bone marrow |
— |
4 |
|
Peripheral blood |
100 |
96 |
Results
Of 160 patients that were initially randomized, 151 underwent allo-HSCT. The median follow-up of transplanted patients was 54.3 months and 56.4 months in the CsA/MPA arm and PTCy/CsA arm, respectively.
There was no significant difference seen in PG180, the primary endpoint of the study (Table 2). Patients in the CsA/MPA arm had significantly reduced GRFS (hazard ratio [HR], 0.5; 95% confidence interval [CI], 0.34–0.74; p<0.001). Improvement of GRFS following PTCy/CsA was irrespective of donor type.
Table 2. Primary and secondary study endpoints after allo-HSCT*
|
allo-HSCT, allogeneic hematopoietic stem cell transplantation; CI, confidence interval; CMV, cytomegalovirus; CsA/MPA, cyclosporine A and mycophenolic acid; GvHD, graft-versus-host disease; GRFS, graft-versus-host disease-free, relapse-free survival; NRM, non-relapse mortality; OS, overall survival; PFS, progression-free survival; PG180, non-severe GvHD within 180 days posttransplant; PT, posttransplant; PTCy/CsA posttransplant cyclophosphamide and cyclosporine A; SE, standard error. |
|||
|
Variable |
CsA/MPA |
PTCy/CsA |
p value |
|---|---|---|---|
|
PG180, % |
29 |
38 |
0.26 |
|
Acute GvHD, % |
65 |
65 |
1.00 |
|
Grade I |
10 |
31 |
|
|
Grade II |
40 |
27 |
|
|
Grade III |
12 |
5 |
|
|
Grade IV |
4 |
1 |
|
|
Chronic GvHD, % |
69 |
49 |
0.025 |
|
Limited |
17 |
25 |
|
|
Extensive |
52 |
24 |
|
|
NRM (at 3 years PT), % (SE) |
14 (5) |
10 (3) |
0.51 |
|
Relapse at 3 years PT, % (SE) |
24 (6) |
32 (5) |
0.27 |
|
Survival outcome estimates, % (95% CI) |
|
|
|
|
3-year PFS |
63 (48–74) |
59 (48–68) |
0.57 |
|
3-year OS |
71 (56–81) |
65 (54–73) |
0.48 |
|
1-year GRFS† |
21 (11–33) |
45 (35–55) |
<0.001 |
The cumulative incidences of aGvHD and cGvHD are shown in Table 3. Multivariate analysis revealed immunosuppression with PTCy/CsA versus CsA/MPA was associated with a reduction in aGvHD Grade II-IV (HR, 0.48; 95% CI, 0.29–0.82; p = 0.007), as well as a reduction in extensive cGvHD (HR, 0.36; 95% CI, 0.21–0.64; p<0.001).
Table 3. Cumulative incidence of acute and chronic GvHD*
|
CsA/MPA, cyclosporine A and mycophenolic acid; GvHD, graft-versus-host disease; PT, posttransplant; PTCy/CsA posttransplant cyclophosphamide and cyclosporine A. |
|||
|
Cumulative incidence, % (SE) |
CsA/MPA |
PTCy/CsA |
p value |
|---|---|---|---|
|
Acute GvHD Grade II–V |
48 (7) |
30 (5) |
0.007 |
|
Acute GvHD Grade III–IV |
12 (4) |
6 (2) |
0.14 |
|
Chronic GvHD (2 years PT) |
65 (7) |
43 (5) |
|
|
Extensive |
48 (7) |
16 (4) |
<0.001 |
Adverse events are summarized in Table 4. A higher incidence of infection, mainly due to increased incidence of febrile neutropenia, was seen in the PTCy/CsA arm.
Table 4. Adverse events*
|
CMV, cytomegalovirus; CsA/MPA, cyclosporine A and mycophenolic acid; GvHD, graft-versus-host disease; PTCy/CsA posttransplant cyclophosphamide and cyclosporine A. |
|||
|
Grade III–V adverse events† <6 months posttransplant, % |
CsA/MPA |
PTCy/CsA (n = 99) |
p value |
|---|---|---|---|
|
All events |
42 |
61 |
0.039 |
|
Infections |
21 |
41 |
0.019 |
|
Febrile neutropenia |
15 |
25 |
0.22 |
|
Invasive pulmonary aspergillosis |
4 |
4 |
1.00 |
|
Other pulmonary infections |
— |
3 |
0.55 |
|
CMV disease |
— |
1 |
1.00 |
|
Graft failure |
2 |
1 |
1.00 |
|
Cardiac |
4 |
3 |
1.00 |
Conclusion
When compared to standard immunosuppression, PTCy/CsA prophylaxis resulted in significantly improved GRFS, and significantly lower incidence of aGvHD (Grade II–IV) and extensive cGvHD, without significantly impacting cumulative relapse rates. Both arms had an acceptable toxicity profile. Severe cGvHD and its ongoing treatment is associated with diminished quality of life, and improved prevention has been shown to increase quality of life scores. Therefore, Broers and colleagues suggest that PTCy prophylaxis may result in an increased quality of life; however, future studies of PTCy should consider patient-reported outcome measures of quality of life to confirm this.
The authors note some potential limitations of their study. The study initially set out to compare standard duration of immunosuppression versus time-restricted immunosuppression; the addition of the PTCy arm occurred later and therefore the primary endpoint should have been adapted to reflect this change. Also, different conditioning regimens were used between the two groups; in the CsA/MPA arm, conditioning was at the discretion of the treating physician, and more intensified conditioning was used in the PTCy/CsA arm. It is presumed that the higher incidence of febrile neutropenia seen in the PTCy/CsA arm is because of this intensified conditioning regimen, although further study of posttransplant infection would be prudent. Additionally, the incidence of cGvHD was reported according to the Seattle classification because at the time of initiation of the study, the National Institutes of Health (NIH) classification was published but was not yet standard in the European transplantation programs. Furthermore, patients received non-myeloablative conditioning regimens; therefore, findings should be considered with caution in allo-HSCT using reduced-intensity or myeloablative conditioning regimens and further study is warranted.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
Which consideration most strongly guides your decision to escalate therapy in SR-aGvHD?



