All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional.
The gvhd Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the gvhd Hub cannot guarantee the accuracy of translated content. The gvhd and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The GvHD Hub is an independent medical education platform, sponsored by Medac and supported through grants from Sanofi and Therakos. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View GvHD content recommended for you
Full dose MTX + Tac vs mini-MTX + Tac/MMF for GvHD prophylaxis
Combination therapy comprising tacrolimus (Tac), a calcineurin inhibitor, and methotrexate (MTX) is the current standard-of-care treatment for graft-versus-host disease (GvHD) prevention after allogeneic hematopoietic cell transplantation.1 However, methotrexate has been associated with toxicities, which can require dose limitations and lead to reductions in efficacy.1 Several other treatments investigated for GvHD prophylaxis have failed to improve outcomes in patients post-HSCT1; however, a study by Mizumoto et al. suggested that a combination of Tac, mycophenolate mofetil (MMF), and reduced dose MTX (mini-MTX) can be used safely and effectively as GvHD prophylaxis.2
This trial is the first to compare the safety and efficacy of Tac/full-dose MTX (full-MTX) and Tac/mini-MTX/mycophenolate mofetil (MMF).1
Study design1
This phase III randomized single-center trial enrolled 101 patients, with 96 included in the final analysis. Of these, 47 patients received the mini-MTX regimen and 49 received the full-MTX regimen. The study design and key inclusion/exclusion criteria is shown in Figure 1.
The primary endpoints of this study were incidence of acute GvHD (aGvHD) by Day 100 (including incidence and severity of Grade 2–4 and Grade 3–4 aGVHD), incidence and severity of mucositis by Day 28, and incidence of neutrophil/platelet engraftment by Day 28. Key secondary endpoints included chronic GvHD, organ toxicity, infection, relapse, non-relapse mortality, and overall survival.
Figure 1. Study design*
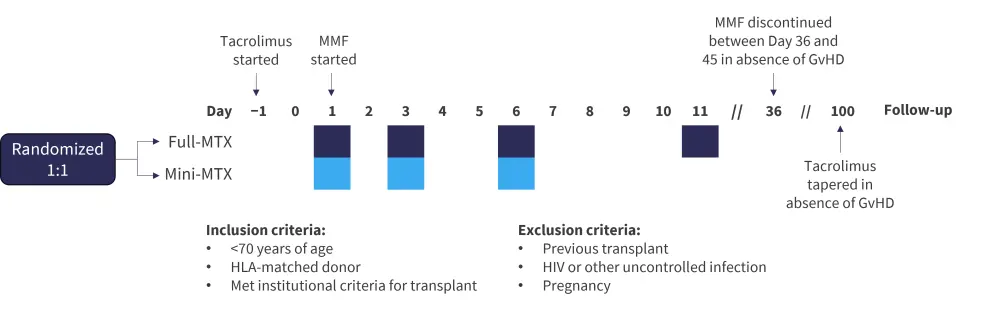
GvHD, graft-versus-host disease; HLA, human leukocyte antigen; MMF, mycophenolate mofetil; MTX, methotrexate.
*Data from Hamilton, et al.1
†Day 1 dose was 15 mg/m2 which was reduced to 10 mg/m2 for following doses. Reductions or dose holds occurred per institutional guidelines for renal/liver toxicities and per physician for mucositis.
‡Dose was 5 mg/m2 for all doses. Reductions or dose holds occurred per institutional guidelines for renal/liver toxicities, and per physician for mucositis.
Results1
Key patient characteristics are shown in Table 1. The majority of patients were white, apart from one black patient in the full-MTX group. Average follow-up time was 19.9 months.
Table 1. Baseline patient characteristics*
|
Bu, busulfan; Cy, cyclophosphamide; MMF, mycophenolate mofetil; MTX, methotrexate; Tac, tacrolimus; tac, tacrolimus; TBI, total body irradiation; VP, etoposide. |
|||
|
Characteristic, % (unless otherwise stated) |
Full-MTX† |
Mini-MTX‡ |
p value |
|---|---|---|---|
|
Sex |
|
|
0.04 |
|
Male |
42.9 |
63.8 |
|
|
Female |
57.1 |
36.2 |
|
|
Median age at transplant (range), years |
47 (5–59) |
45 (2–62) |
0.47 |
|
Donor |
|
|
0.56 |
|
Matched unrelated |
77.6 |
72.3 |
|
|
Matched sibling |
22.4 |
27.7 |
|
|
Conditioning |
|
|
0.54 |
|
Bu/Cy |
81.6 |
83.0 |
|
|
TBI/VP |
18.4 |
14.9 |
|
|
Cy/TBI |
0 |
2.1 |
|
|
MTX doses |
|
|
<0.001 |
|
2 |
2.0 |
0 |
|
|
3 |
26.5 |
100.0 |
|
|
4 |
71.4 |
0 |
|
Efficacy
There were no significant differences in GvHD outcomes between the groups. GvHD outcomes at Day 100 (incidence of Grade 2–4 aGvHD and Grade 3/4 aGvHD) and Month 12 (incidence of moderate/severe chronic GvHD) are shown in Figure 2.
Figure 2. Cumulative incidence of GvHD outcomes*
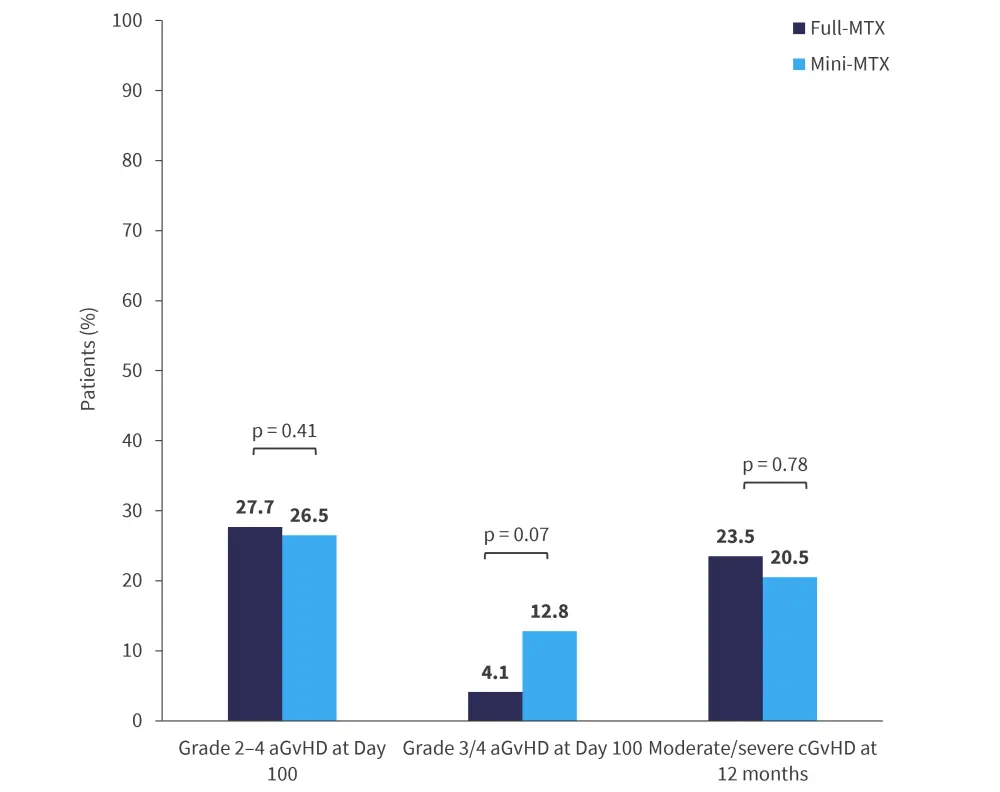
aGvHD, acute graft-versus-host disease; cGvHD, chronic GvHD; MTX, methotrexate.
*Adapted from Hamilton, et al.1
Although relapse rates differed between groups, these differences were not significant; the study was not powered to detect statistical differences in survival outcomes. The most common cause of death in both groups was relapse (full-MTX, 50%; mini MTX, 67%). Survival and relapse outcomes are shown in Figure 3.
Figure 3. Survival and relapse outcomes at 12 months*
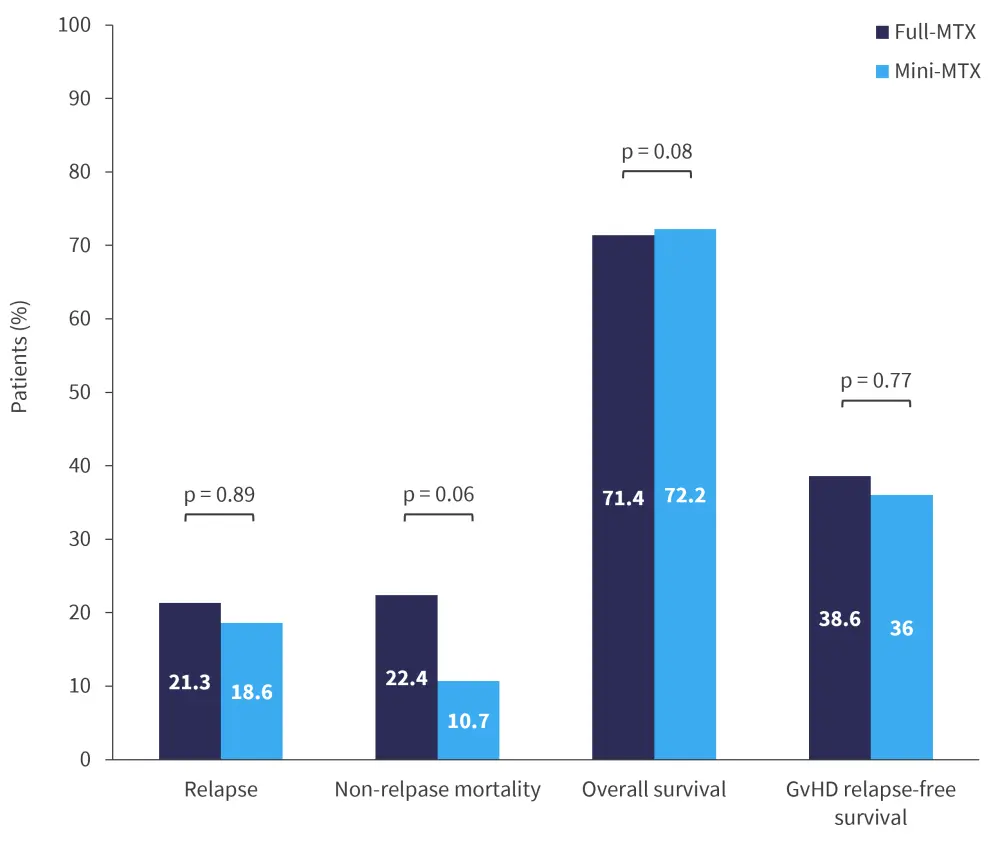
GvHD, graft-versus-host disease; MTX, methotrexate.
*Adapted from Hamilton, et al.1
Engraftment failure occurred in one patient who received full-MTX. Neutrophil and platelet engraftment was significantly faster in patients who received mini-MTX compared with full-MTX (15 days vs 17 days and 23 days vs 28 days, respectively); therefore, patients who received mini-MTX had a significantly shorter duration of hospitalization than those who received full-MTX (27 days vs 31 days).
Safety
Key safety results for full-MTX vs mini-MTX treated patients included:
- significantly higher World Health Organization (WHO) Grade 3–4 mucositis (82% vs 57%; p = 0.01);
- significantly higher median duration of mucositis (18 days vs 11 days; p < 0.001);
- no significant difference in use of patient-controlled analgesia; and
- no significant difference in the rate of infections.
Conclusion
This study demonstrated that mini-MTX can result in significantly less mucositis and significantly faster neutrophil and platelet engraftment compared to full-MTX.1 However, the primary endpoint of aGvHD incidence at Day 100 was not significantly different between the two groups.1
The study was not powered to detect statistical differences in survival outcomes; however, non-relapse mortality was lower in patients who received mini-MTX.1 Although his study suggests that mini-MTX can be safe and effective in patients undergoing transplant, additional studies in larger and more varied patient cohorts are needed. Tac/MTX was used in the majority of human leukocyte antigen matched-related (63%) and unrelated (64%) transplants between 2018 and 2020, it will likely remain the standard-of-care GvHD prophylaxis.1
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
Which consideration most strongly guides your decision to escalate therapy in SR-aGvHD?


