All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional.
The gvhd Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the gvhd Hub cannot guarantee the accuracy of translated content. The gvhd and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The GvHD Hub is an independent medical education platform, sponsored by Medac and supported through grants from Sanofi and Therakos. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View GvHD content recommended for you
Editorial theme | Considerations for allo-HSCT from mismatched unrelated donors
Allogeneic hematopoietic stem cell transplantation (allo-HSCT) is very commonly used for the treatment of hematological malignancies. With the help of immunogenomics, precise mapping of the expressed human leukocyte antigen (HLA) haplotypes is routinely performed for the identification of the closest matching donor and thus the maximisation of allo-HSCT outcomes, in terms of graft-versus-host disease (GvHD) and survival.3
HLA-matched sibling donors are the ideal donors, but they are only available in approximately 30% of cases.1 The next best option is an HLA-matched unrelated donor (MUD), which is also not always available, especially for patients of non-European background2, leading to the choice between HLA-mismatched related or unrelated donors (MMUD), HLA-haploidentical (HD) donors, or umbilical cord blood (Figure 1).3,4
Although other stem cell sources may be preferred over MMUDs, significant advances and research has provided further insights into what is needed to maximise the efficacy and tolerability of MMUDs for patients in need. We hereby provide a mini review on the current considerations regarding MMUD allo-HSCTs. For more information on the other donor types (HLA-matched sibling donors, MUD, HD, and umbilical cord blood) and their comparative impact on allo-HSCT outcomes, including MMUDs, read this article.
Figure 1. Donor types for allogeneic hematopoietic stem cell transplantation
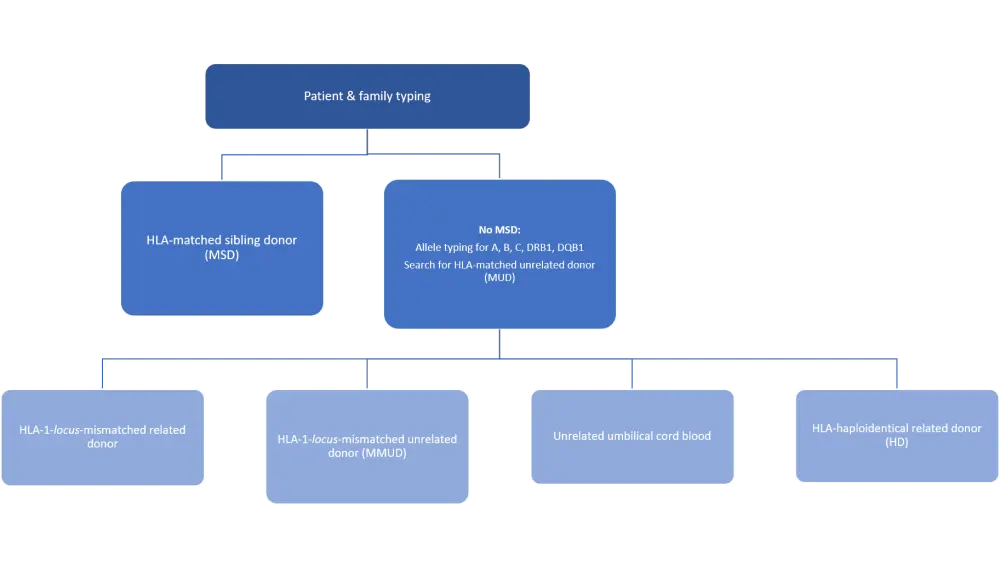
Donor types are shown in order of best transplantation efficacy and risk minimization.
HLA, human leukocyte antigen
Allo-HSCT outcomes depend on the number and specificity of HLA mismatches
Three class I genes, HLA-A, -B, and -C, and three class II genes, HLA-DR, -DQ, and -DP, on the human major histocompatibility complex locus are the allo-HSCT determinants.2 MMUDs have been commonly defined by one or two HLA mismatches in the most commonly mapped loci, like HLA-A, -B, -C, and -DRB1.4 Recently, other loci, like DPB1 and DQB1, are also taken into consideration when allele typing for MMUDs. The total number of HLA mismatches between donor and recipient in those loci with high expression significantly affects the overall survival of allo-HSCT patients, with overall survival rates between 8/8 MUD, 7/8 MMUD, and 6/8 MMUDs, declining stepwise from 52% to 43% to 33%, respectively.4 Similarly, a stepwise increase in acute GvHD (aGVHD) has also been reported among these three donor groups, with a higher number of HLA mismatches leading to a higher risk of Grade 2–4 aGvHD.2
Specific HLA mismatches increase the risk of GvHD, while others may be associated with a lower risk of posttransplant recurrence due to graft-versus-leukemia effect.3 More specifically, HLA-A, -B, or -C mismatches are associated with a higher risk of GvHD and transplant-related mortality.3,11 Less routinely typed alleles, like HLA-DQB1, may also be important for risk minimization. Mismatches at HLA-DQB1 alone do not appear to increase GvHD risk or affect allo-HSCT outcomes, but the existence of both HLA-DQB1 and -DRB1 mismatches significantly increase the risk of GvHD.3
Early studies on HLA-DPB1 indicated that mismatches at this locus are associated with increased GvHD but do not impact on the overall survival of patients or the transplant-related mortality rates. As a result, HLA-DPB1 typing was not considered in the past.11 Although HLA-DPB1 mismatches increase GvHD risk, a recent study showed that they are accompanied by a lower risk of relapse.5,11 More information about this study can be found here.5 The precise significance of HLA-DPB1 in informing GvHD risk was recently investigated by Petersdorf et al. in a large retrospective study (N = 19,136) published in the Journal of Clinical Oncology.6 The results showed that, in patients receiving MMUD with a mismatch in HLA-A, -B, -C, DRB1, or -DQB1, the odds of aGvHD increased relative to increasing numbers of HLA-DPB1 mismatches but not relative to its expression level.6 These data indicate that the HLA-DPB1 number of mismatches, but not expression level, can be used to inform aGvHD risk in MMUDs and highlight the importance of considering HLA-DPB1 in allele typing for MMUDs. Interestingly, the same study also shows that the level of HLA-DPB1 mismatch expression level informs aGvHD risk in HLA-A, -B, -C, -DRB1, and -DQB1-matched unrelated allo-HSCT.6
Another recent study, published by Petersdof et al. in Blood, shows that MMUD outcomes depend on the dimorphism in the HLA-B leader peptide and the mismatched HLA locus, providing a novel extra consideration when undertaking MMUDs.7 More specifically, the authors of this large retrospective study (N = 11,872) show that HLA-DQB1-mismatched patients with the HLA-B methionine–methionine (MM) dimorphism had higher non-relapse mortality (NRM) rates than the same group of patients but with the threonine–threonine HLA-B dimorphism.7 The same applied to Grade 3–4 GvHD risk, which was higher in patients with HLA-DRB1 mismatch and methionine–methionine or methionine–threonine dimorphism compared to those with HLA-DRB1 mismatch threonine–threonine dimorphism.7 The results of this study indicate that HLA-B dimorphism type can be used in combination with the HLA locus mismatch to inform GvHD or NRM risk in MMUDs.
To summarize, a maximum of one HLA mismatch is allowed for commonly mapped loci in order to increase overall survival and reduce GvHD after transplantation. For other loci, like HLA-DRB3, -DRB4, -DRB5, or -DQB1, the aim is to match at least four of them, as the presence of three or more low-expression mismatches increases overall mortality and transplantation-related mortality, even in 7/8-matched MMUDs2. New considerations to be taken into account for MMUDs include the identification of exact HLA-B dimorphism in HLA-DRB1 or -DQB1-mismatched cases and the use of the HLA-DPB1 number of mismatches for GvHD risk prognosis. Table 1 summarizes the key considerations regarding HLA matching and haplotypes when undertaking a MMUD allo-HSCT.
Table 1. Summary of considerations for MMUD allo-HSCT regarding HLA mismatching2,6,7
|
MMUD considerations regarding HLA mismatching |
|---|
|
(a)GvHD, (acute) graft-versus-host disease; HLA, human leukocyte antigen; MMUD, mismatched unrelated donor; NRM, non-relapse mortality |
|
|
|
|
|
Other donor considerations
Donor age and sex have been reported to influence GvHD incidence and survival outcomes following MMUD allo-HSCT. The ideal donor candidate for MMUD are males ≤ 33 years old, as multi-parity in women has been linked to higher chronic GvHD (cGvHD) risk.4 The recommended stem cell source from the donor has been identified as the bone marrow with a minimum of 3 × 108/kg total nucleated cells.4 If the donor’s blood is not ABO compatible to the recipient, then the bone marrow stem cell graft should increase to 4 × 108/kg total nucleated cells.4 The presence of donor-specific anti-HLA antibodies in the recipient precludes the use of the graft, and another one should be considered to minimize the risk of engraftment failure.4
GvHD prophylaxis and conditioning for MMUD
Antilymphocyte globulin (ATG) has been used effectively as GvHD prophylaxis for patients undergoing allo-HSCT. However, its use has been linked to delayed T cell reconstitution, that in turn increases the incidence of infection and/or relapse.3,8,9 There are limited retrospective trials that have evaluated the role of ATG in MMUDs, as most patients usually receive MUD or MSD.3 Moreover, the efficacy of posttransplant cyclophosphamide (PtCy), an efficient GvHD prophylactic in HD transplantations, has not been extensively studied in MMUDs.3,8,9 Two recent retrospective studies compared PtCy to ATG in patients undergoing MMUD allo-HSCT. In both studies, PtCy was associated with significantly improved patient outcomes and a lower rate of Grade 3–4 aGvHD when compared to ATG.8,9 These results indicate that PtCy is good alternative to ATG in the MMUD setting. More large retrospective trials are needed to further validate these results. For more information on the study designs and outcomes of these trials, read this article.
A recent phase III trial evaluated the addition of sirolimus (S) to cyclosporine and mycophenolate mofetil (CMM) as GvHD prophylaxis for patients receiving non-myeloablative conditioning for MMUD allo-HSCT.10 The cumulative incidence of Grade 2–4 aGvHD was significantly lower when sirolimus was added to CMM (CMMS), including a marked reduction in aGvHD presenting in the skin. No differences between CMMS and CMM were observed in terms of cGvHD incidences, but more patients in the CMM arm progressed from aGvHD to cGvHD.10 These results showed that CMMS combination is an efficient aGvHD prophylactic in patients receiving non-myeloablative conditioning for MMUDs. For more information on this phase III trial, read this article.
Published expert clinical opinions recommend PtCy, ATG, or alemtuzumab for GvHD prophylaxis in MMUDs, and deploying a conditioning regimen that matches these anti-GvHD agents. More specifically, if PtCy is used, mycophenolate mofetil with tacrolimus or sirolimus should be added, while if alemtuzumab is administered, tacrolimus alone may suffice. For ATG, the current practice is to combine it with methotrexate and/or tacrolimus.4 Nevertheless, at the moment, the best GvHD prophylactic agent for MMUDs is not evident, with divergent clinical opinions because of the lack of sufficient retrospective comparative trials. This lack of specific recommendations is also apparent in the 2020 published consensus from the European Society for Blood and Marrow Transplantation (EBMT) on GvHD prophylaxis and management following HSCT.11 A summary of the 2020 EBMT recommendations can be found here.
Conclusion
MMUDs provide more choice when it comes to allo-HSCT donor options in patients that lack MUD or MSD. The number and type of HLA mismatches are the most important risk factors for treatment failure and GvHD in this setting, thus careful HLA screening and matching is crucial. MMUD transplants can be further optimized by considering the age and sex of the donors, as well as the stem cell source. Bone marrow sampling from young male donors appears to be an optimal graft source. Since GvHD prophylaxis and conditioning is key for MMUD efficacy and patient outcomes, multiple agents are being used in practice, e.g., ATG, PtCy, CMMS, and tacrolimus. Nevertheless, the comparative efficacy of anti-GvHD agents and the identification of the optimal one needs further clinical exploration in large retrospective trials specific to the MMUD setting.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
Which consideration most strongly guides your decision to escalate therapy in SR-aGvHD?

