All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional.
The gvhd Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the gvhd Hub cannot guarantee the accuracy of translated content. The gvhd and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The GvHD Hub is an independent medical education platform, sponsored by Medac and supported through grants from Sanofi and Therakos. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View GvHD content recommended for you
The use of Day 14 endpoint models in aGvHD clinical trials
The overall response rate (ORR) of clinical symptoms at Day 28 is a validated replacement for non-relapse mortality (NRM) and has been accepted as the primary endpoint for clinical trials of acute graft-versus-host disease (GvHD) treatment.1 Physicians typically modify immunosuppression earlier than Day 28 for patients who do not respond to treatment; therefore, measuring ORR at Day 28 may not be as effective.1 A further limitation of using Day 28 ORR is that it does not account for the distinct treatment goals required for patients with high- and low-risk GvHD.1
Here, we summarize an article by Spyros et al.1 published in Transplantation and Cellular Therapy, which evaluates Day 14 endpoint models for acute GvHD (aGvHD) as predictors of long-term outcomes.
Methods1
- Patients who underwent hematopoietic cell transplantation between January 2015 and December 2021, and had data and samples in the Mount Sinai Acute GvHD International Consortium (MAGIC) database and biorepository were evaluated.
- Data were collected and reviewed using a prospective-specimen collection, retrospective-blinded-evaluation (PRoBE) design.
- Patients with Grade 1─4 aGvHD, who had not relapsed, and were treated with 0.25 mg/kg/day of systemic steroids or other immunosuppressive agents were included in the analysis.
- A recursive partitioning classification tree algorithm was used to develop a new predictive model (the Day 14 Mount Sinai model), which accounts for the grade of GvHD at treatment initiation and at Day 14 to classify patients into favorable and unfavorable groups, with 12-month NRM as the outcome variable.
- The Day 14 MAGIC algorithm probability (MAP) score was included in the Day 14 Mount Sinai model to create a Day 14 MAGIC model, using Day 14 MAP biomarker score (low ≤ 0.290 vs high > 0.290).
- Responses or non-responses at Day 28 were categorized according to standard clinical criteria.
- Quantitative comparisons were made of the different response models for censored event times with competing risks.
Key findings1
- In total, 1,144 patients, from 23 MAGIC sites, were included in this study.
- The Mount Sinai model correctly identified 57% of NRM events in the unfavorable group compared with 48% identified by the Day 28 standard criteria model in the non-response group.
- With the Day 14 MAGIC model, three distinct response populations were identified which were good, intermediate, and poor, with escalating NRM (8%, 35%, and 76%, respectively).
- With Day 28 standard criteria, partial and complete responders experienced overlapping NRM.
- The Day 14 MAGIC model successfully stratified patients with Grade 2 aGvHD at onset that remained Grade 2 at Day 14.
- Overall, 12 months following GvHD treatment, the Day 14 MAGIC model was more accurate, as measured by area under the curve vs the Day 28 standard clinical response and the Day 14 Mount Sinai model (Figure 1).
Figure 1. Quantitative comparison of Day 28 and Day 14 models for competing risks with NRM as outcome*
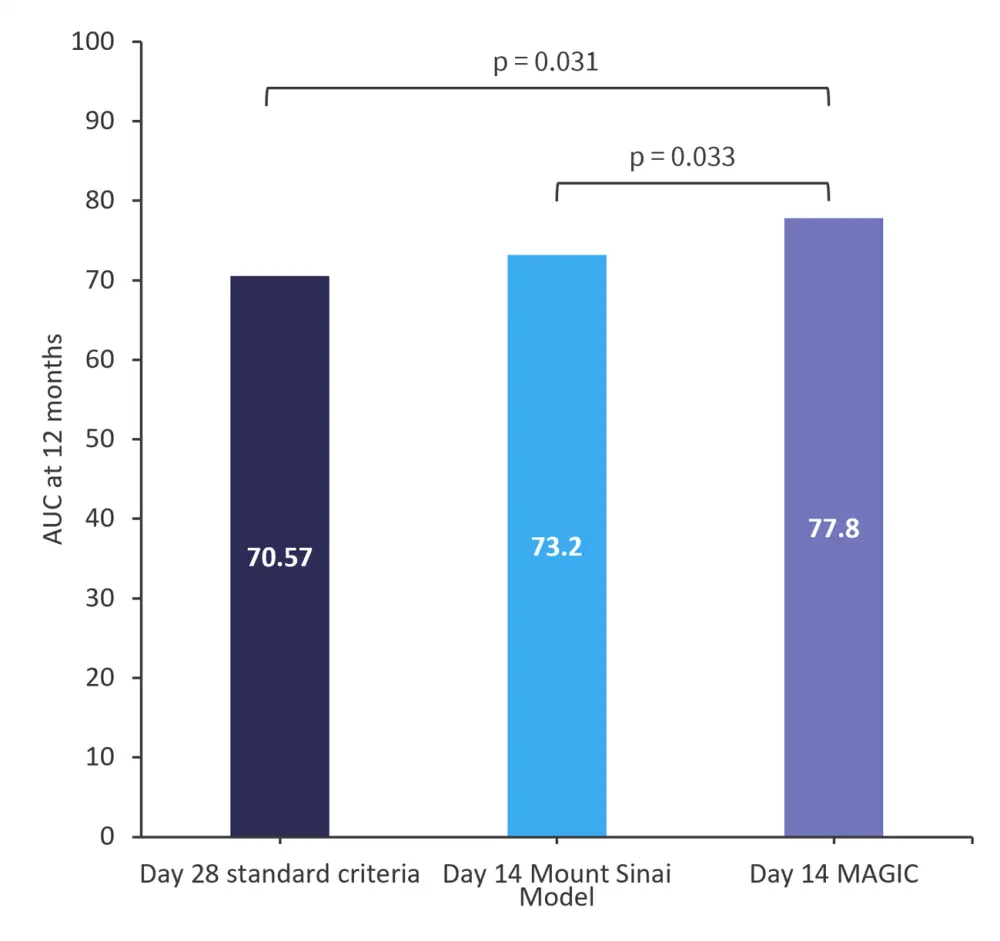
AUC, area under the curve; GvHD, graft-versus-host disease; NRM, non-relapse mortality; MAGIC, Mount Sinai Acute GvHD International Consortium.
*Data from Spyrou, et al.1
- The Day 14 MAGIC model displayed the most favorable sensitivity, Youden’s index, and negative predictive values among the models when 12-month NRM was considered as the endpoint (Table 1).
Table 1. Model characteristics at the 12-month post-treatment NRM endpoint*
|
Model |
Sensitivity |
Specificity |
Youden’s index |
NPV |
|
GvHD, graft-versus-host disease; MAGIC, Mount Sinai Acute GvHD International Consortium; NPV, negative predictive values; NRM, non-relapse mortality; ORR, overall response rate. |
||||
|
Day 14 standard criteria (ORR) |
0.54 |
0.77 |
0.31 |
0.88 |
|
Day 28 standard criteria (ORR) |
0.54 |
0.87 |
0.41 |
0.89 |
|
Day 14 Mount Sinai model |
0.57 |
0.90 |
0.47 |
0.90 |
|
Day 14 MAGIC model |
0.71 |
0.85 |
0.56 |
0.92 |
|
Key learnings |
|
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
Which consideration most strongly guides your decision to escalate therapy in SR-aGvHD?


