All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional.
The gvhd Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the gvhd Hub cannot guarantee the accuracy of translated content. The gvhd and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The GvHD Hub is an independent medical education platform, sponsored by Medac and supported through grants from Sanofi and Therakos. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View GvHD content recommended for you
The safety and efficacy of fecal microbiota therapy in patients with GvHD
The gastrointestinal tract and associated gut microbiota, together forming the largest immune organ in the human body, are associated with the development of acute graft-versus-host disease (aGvHD).1
Patients who have previously received hematopoietic stem cell transplantation (HSCT) can often present with gut dysbiosis and reduced diversity in stool bacteria. GvHD can also negatively impact gut health, leading to translocation of bacteria into the blood and worsening dysbiosis.1
Fecal microbiota therapy (FMT), which involves the transplant of fecal microbiota from healthy donors to the gut of HSCT patients, has been used to combat this problem with promising results. However, the recent clinical hold by the U.S. Food and Drug Administration (FDA) of MaaT013, a pooled allogeneic fecal microbiotherapy product, has raised questions about the safety and efficacy of FMT. The GvHD Hub has previously reported on MaaT013 and the Heracles trial (NCT03359980) in patients with steroid-refractory aGvHD. Here, we discuss a systemic review of 23 clinical trials, studies, and case reports of FMT in patients with acute or chronic GvHD by Bilinski et al.1 published in Bone Marrow Transplant in 2022.
Study details
A total of 23 studies including 242 patients with GvHD were included in the meta-analysis, of which 161 responded to FMT, 100 achieved complete response, and 61 achieved a partial response. Table 1 outlines seven of the studies (those with the highest patient number per route of administration). The remaining studies comprised two on FMT by capsule and five on nasoduodenal tube or nasogastric tube; the remainder used a combination of administration routes.
Table 1. Details of seven FMT studies*
|
EAP, early access program; FMT, fecal microbiota transplantation; GI, gastrointestinal; N/A, not available; NT, nasoduodenal tube or nasogastric tube; SR-aGvHD, steroid-refractory acute graft-versus-host disease. |
|||||||
|
Study |
Study type |
Disease type |
n |
Age range, years |
Route of administration |
Donor type |
FMTs, n† |
|---|---|---|---|---|---|---|---|
|
1. Shouval, et al. (NCT03214289) |
Single-arm pilot study |
SR-aGvHD (GI) |
7 |
41–72 |
Capsules (frozen) |
Unrelated |
1–3 |
|
2. Goeser, et al. |
Retrospective study |
SR-aGvHD (GI) |
11 |
30–76 |
NT, capsules |
2 related, 9 unrelated |
1 |
|
3. Spindelboeck, et al. (NCT03819803) |
Prospective cohort study |
SR-aGvHD |
9 |
24–67 |
Colonoscopy |
6 unrelated, 3 related |
1–6 |
|
4. Malard, et al. (NCT03359980) |
Phase II trial (HERACLES) |
SR-aGvHD (GI) grade III-IV |
24 |
N/A |
MaaT013 |
Unrelated |
N/A |
|
5. Malard, et al. |
EAP |
SR-aGvHD (GI) grade II-IV |
52 |
N/A |
MaaT013 |
Unrelated |
1–6 |
|
6. van Lier, et al. (ISRCTN14530574) |
Prospective study |
SR-aGvHD (GI) |
15 |
20–72 |
NT (fresh) |
13 unrelated, 2 related |
1 |
|
7. Bilinski, et al. |
Phase I/II trial |
SR-aGvHD (GI) |
13 |
13–55 |
NT (fresh) |
Unrelated |
1–2 |
Study findings
Safety
Adverse events reported across the 23 studies included infections, nausea, vomiting, abdominal pain, diarrhea, and constipation. Routine post-HSCT immunosuppression resulted in an increased risk for microbiota translocation and infections, with higher rates in patients who received FMT to treat C. difficile. Across all studies, five infections were severe and 90 deaths were reported, with none relating at FMT.1
Efficacy
Response rates varied between the studies (Figure 1). Similar efficacy was seen in capsules, colonoscopy, and MaaT013, although patients treated through a nasoduodenal tube or nasogastric tube showed higher response rates.1 However, Goeser et al. stated that capsules led to less discomfort and improved safety compared with more invasive methods.2 It is important to note that there was an over-representation of FMT by nasoduodenal tube or nasogastric tube, so efficacy cannot be directly compared to other administration routes.
Figure 1. Response rates*
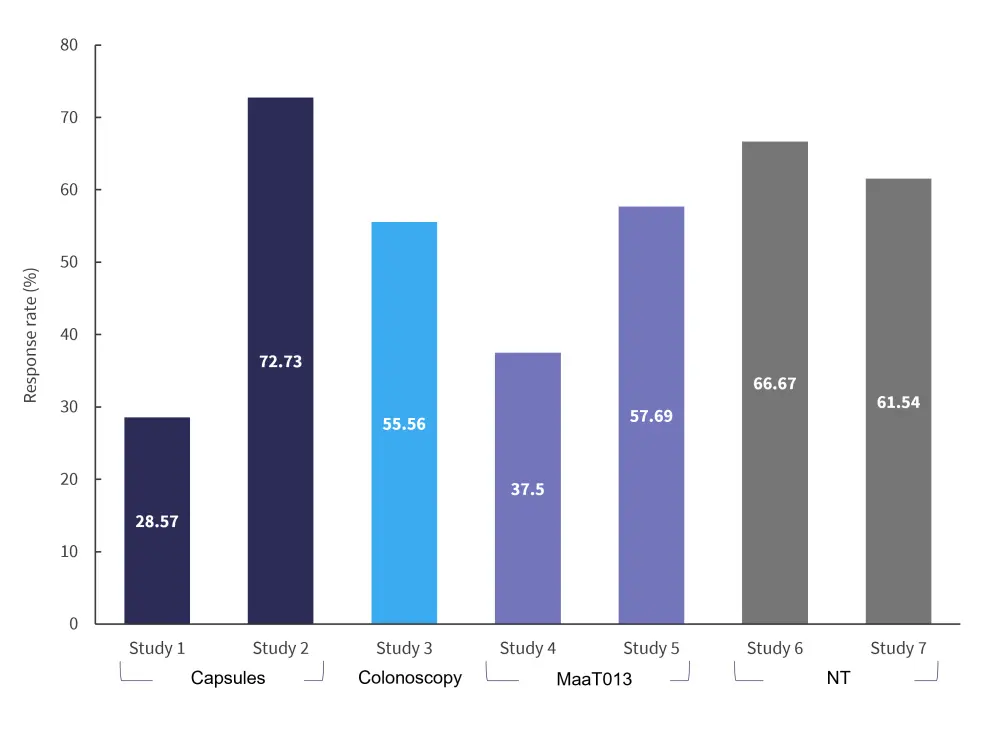
NT, nasoduodenal tube or nasogastric tube.
*Data from Bilinski, et al.1
Conclusion
FMT appears to reduce GvHD due to reconstitution of the gut microbiota; however, the exact mechanism is yet to be elucidated. After FMT, there was a dominance of beneficial gut bacteria in patients with mitigating GvHD. Conversely, in patients whose GvHD relapsed after FMT, there was a dominance of bacteria correlated with GvHD, such as enterococci and proteobacteriaceae. In addition, a healthy gut microbiome can produce anti-inflammatory metabolites which increase expression of interleukin-10 and interleukin-17, reducing GvHD symptoms. The majority of adverse events experienced by patients were mild or moderate, with only five severe infections and no deaths were reported due to FMT. A limitation of this meta-analysis was a lack of the larger, randomized, placebo-controlled trials needed to fully assess the overall safety and efficacy of FMT. Although FMT appears to be a promising treatment for patients with GvHD, future trials comparing route and frequency of FMT in larger sample sizes are needed to confirm these findings.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
Which consideration most strongly guides your decision to escalate therapy in SR-aGvHD?


