All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional.
The gvhd Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the gvhd Hub cannot guarantee the accuracy of translated content. The gvhd and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The GvHD Hub is an independent medical education platform, sponsored by Medac and supported through grants from Sanofi and Therakos. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View GvHD content recommended for you
Update from the Heracles trial and expanded access program of MaaT013 in patients with SR aGvHD
Gut dysbiosis in acute graft-versus-host disease (aGvHD) is associated with poor outcomes. Fecal microbiota transplant has been assessed as a potential treatment for patients with gastrointestinal (GI) aGvHD; however, the issue of donor sourcing of microbiota remains. One potential solution is the creation of a standardized fecal microbiota transplant product. This is the idea behind MaaT013, a pooled allogeneic fecal microbiotherapy product. The results of the expanded access program (EAP) have been reported on previously by the GvHD Hub here.
During the 63rd American Society of Hematology (ASH) Annual Meeting & Exposition and the 47th Annual Meeting of the European Society for Blood and Marrow Transplantation (EBMT), Florent Malard1,2 discussed the results of MaaT013 in the Heracles phase IIa trial (NCT03359980) and the EAP in patients with steroid refractory (SR) aGvHD.
Study design
Heracles trial
Figure 1. Study design, including eligibility and exclusion criteria*
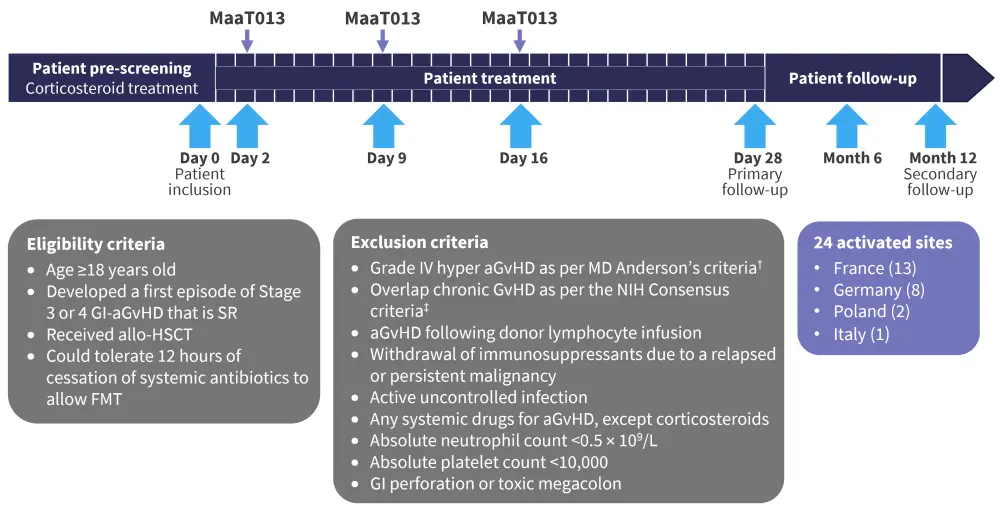
aGvHD, acute graft-versus-host disease; allo-HSCT, allogeneic hematopoietic stem cell transplant; FMT, fecal microbiota transplant; GI, gastrointestinal; NIH, National Institutes of Health; SR, steroid refractory
*Adapted from Malard.1
†Saliba et al.3
‡Jagasia et al.4
Expanded access program
The EAP allowed patients to be treated who were not eligible for the Heracles trial. This included:
- patients with SR or steroid dependent aGvHD of any grade with GI involvement; and
- patients on any line of treatment.
Results
Patient characteristics were similar between the two cohorts, with a median age of 57 years in the EAP cohort and 61 years in the Heracles cohort. Acute myeloid leukemia was the most common diagnosis, for 44% of the EAP cohort, compared with 38% of the Heracles cohort. In both cohorts, most patients had Grade III aGvHD (Table 1).
Table 1. Patient characteristics*
|
aGvHD, acute graft-versus-host disease; ALL, acute lymphoblastic leukemia; AML, acute myeloid leukemia; EAP, expanded access program; FMT, fecal microbiota transplant; GvHD, graft-versus-host disease; MDS, myelodysplastic syndrome; MM, multiple myeloma; MPN, myeloproliferative neoplasm; TCL, T-cell lymphoma. †Glucksberg criteria. |
||
|
Characteristics, % (unless stated otherwise) |
EAP |
Heracles |
|---|---|---|
|
Median age (range), years |
57 (18−73) |
61 (20−69) |
|
Sex |
|
|
|
Male |
60 |
67 |
|
Female |
40 |
33 |
|
Diagnosis |
|
|
|
AML |
44 |
38 |
|
MDS |
15 |
12 |
|
MPN |
19 |
25 |
|
ALL |
12 |
8 |
|
TCL |
8 |
0 |
|
MM |
2 |
0 |
|
Others |
0 |
17 |
|
Steroid status |
|
|
|
Resistant |
83 |
— |
|
aGvHD grade at the time of FMT request† |
|
|
|
0 |
0 |
0 |
|
I |
0 |
0 |
|
II |
6 |
0 |
|
III |
94 |
96 |
|
IV |
0 |
4 |
|
Organ involvement |
|
|
|
Gut |
100 |
100 |
|
Skin |
32‡ |
54 |
|
Liver |
13 |
25 |
|
Median number of previous aGvHD treatments (range) |
3 (1−6) |
— |
|
Used ruxolitinib before |
77 |
— |
|
Median number of MaaT013 delivered (range) |
3 (1−3) |
— |
Efficacy
In the Heracles trial, 38% of patients achieved a positive GI GvHD response at Day 28, compared with 58% in the EAP. The best overall response until Day 28 was 54% in the Heracles trial and 67% in the EAP (Figure 2).
Figure 2. Outcomes in the Heracles trial compared with EAP*
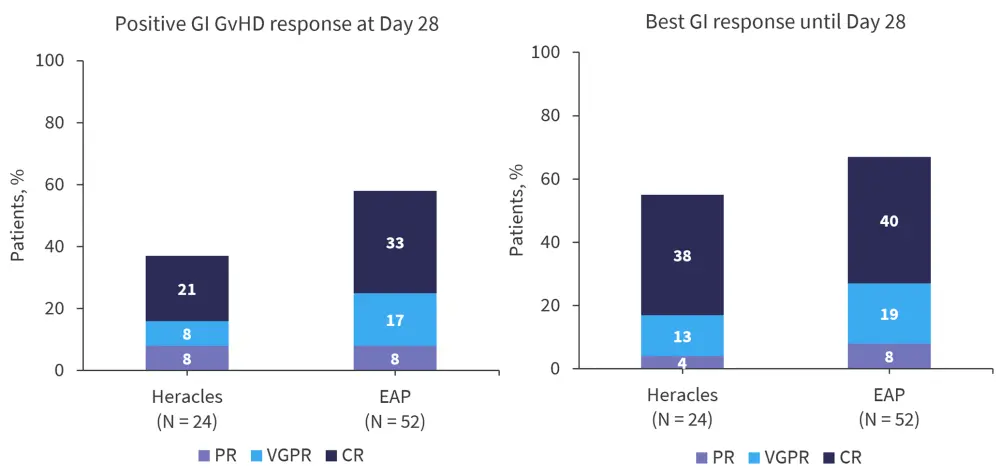
CR, complete response; GI, gastrointestinal; GvHD, graft-versus-host disease; PR, partial response; VGPR, very good partial response.
*Adapted from Malard.1
Overall survival at 12 months was 25% in the Heracles trial and 38% in the EAP. The median follow‑up for the patients who were still alive in the EAP was 361 days (range, 63−731 days) (Table 2). Overall survival at 12 months was significantly increased in the patients who responded compared with non-responders in the Heracles (p = 0.047) and EAP (p < 0.0001) cohorts.
Table 2. Overall survival in the Heracles trial and EAP*
|
CR, complete response; EAP, expanded access program; NR, no response; OS, overall survival; PR, partial response; VGPR, very good partial response. |
||
|
|
EAP (N = 52) |
Heracles (N = 24) |
|---|---|---|
|
OS depending on timepoint, % |
|
|
|
6 months |
49 |
29 |
|
12 months |
38 |
25 |
|
OS at 12 months depending on response category, % |
|
|
|
CR/VGPR/PR |
59 |
44 |
|
NR |
7 |
13 |
Safety
Heracles
Within 24 hours of MaaT013 administration, 39 adverse events (AEs) were reported.
Serious AEs included:
- Two Grade III AEs: Escherichia sepsis (E. coli strain not found in MaaT013 sample received by patient) and thrombotic microangiopathy
- One Grade IV AE: cerebral infarction
- One Grade V AE: decrease in overall physical health
In patients who responded, there was a significantly improved alpha diversity of the patients’ gut microbiome and higher proportions of MaaT013-derived species in the gut microbiota following MaaT013 treatment.
EAP
Following MaT013 administration, GI symptoms (abdominal pain and anorectal disorder) were recorded in two patients. Infectious complications occurred in 11.5% of patients and a connection to MaaT013 could not be ruled out. These included bacterial arthritis, sepsis, Escherichia bacteremia, bacterial translocation, bacteroides infection, and septic shock.
Conclusion
Malard concluded by highlighting that MaaT013 has been used to treat >80 patients so far between the Heracles trial and EAP and demonstrated improved alpha diversity of the gut microbiome. For patients with SR or steroid dependent aGvHD, an overall response rate at Day 28 of 38% was achieved in the Heracles trial and 58% in the EAP. Malard went on to highlight the ongoing ARES phase III single-arm trial (NCT04769895), which aims to assess MaaT013 treatment as a third line treatment in this patient group.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
Which consideration most strongly guides your decision to escalate therapy in SR-aGvHD?

