All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional.
The gvhd Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the gvhd Hub cannot guarantee the accuracy of translated content. The gvhd and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The GvHD Hub is an independent medical education platform, sponsored by Medac and supported through grants from Sanofi and Therakos. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View GvHD content recommended for you
Real-world outcomes of belumosudil compared with best available therapy: Results from the ROCKreal study
Do you know... In the ROCKreal study, 26.8% of patients treated with best available therapy achieved overall response at 6 months. What is the approximate percentage of belumosudil-treated patients who achieved ORR at 6 months?
Belumosudil is a selective inhibitor of Rho-associated coiled-coil-containing protein kinase 2 (ROCK2), a signaling protein involved in inflammation and fibrosis.1 It is approved in the US for the treatment of chronic graft-versus-host disease (cGvHD) after failure of at least two prior lines of systemic therapy.1
The phase II ROCKstar trial (NCT03640481) evaluated belumosudil 200 mg once or twice daily in 132 patients with heavily pre-treated cGvHD; safety and efficacy were assessed for up to 3 years.1 The study found that daily treatment with 200 mg of belumosudil induced responses in 74% of patients, including those with cGvVHD refractory to steroids, ruxolitinib, and ibrutinib.1 However, these positive results only apply to a specific patient population, with strict inclusion and exclusion criteria, and therefore additional real-world data are needed to complement the findings, particularly comparison with current best available therapy (BAT).
ROCKreal study design2
ROCKreal was a non-interventional, chart-review study across eight centers in the US, which imitated a phase III trial through causal inference methodology. Patients aged ≥12 years with cGvHD who had received 2–5 prior lines of therapy between 2015–2024 were included in the study; those previously exposed to belumosudil were excluded.
Figure 1. Study design*
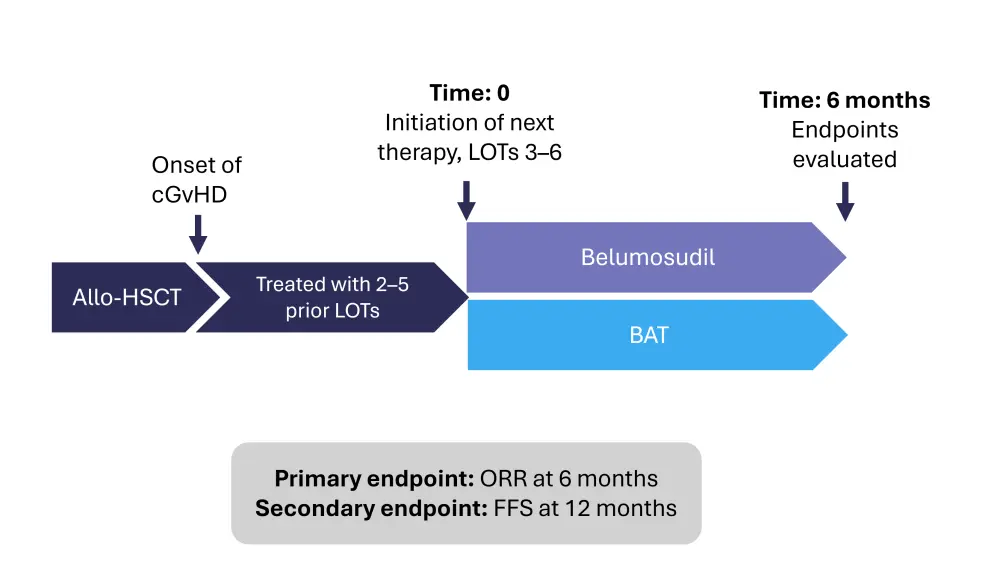
Adjusted overall response rate (ORR) at 6 months was used to assess patient response; a responder was classified as a patient with a complete or partial response, or a 50% reduction in corticosteroids without disease progression. Response failure was defined as mixed response, disease progression, relapse, treatment switching, or death.
Efficacy and safety results
In total, 196 patients were included in the study, of which 113 received belumosudil and 83 BAT, resulting in 358 LOTs (113 belumosudil, 245 BAT). There was a higher median age and number of prior LOTs at baseline in the belumosudil group compared with the BAT group (Table 1).
Table 1. Baseline characteristics for patients in the ROCKreal study*
Characteristic | Belumosudil (LOTs N = 113) | BAT (LOTs N = 245) |
|---|---|---|
Median age at LOT start, years | 63.1 | 55.2 |
Male, % | 58.0 | 59.0 |
Median number of prior LOTs | 3 | 2 |
Median number of organs involved at LOT start | 3 | 3 |
PBSCs graft source | 94.0 | 90.0 |
Conditioning regimen |
|
|
RIC/non-myeloablative | 62.0 | 53.0 |
Myeloablative | 38.0 | 47.0 |
Prior cGvHD therapies |
|
|
ECP | 34.0 | 26.0 |
Ruxolitinib | 68.0 | 36.0 |
Ibrutinib | 24.0 | 20.0 |
BAT, best available therapy; cGvHD, chronic graft-versus-host disease; ECP, extracorporeal photopheresis; LOT, line of therapy; PBSC, peripheral blood stem cell; RIC, reduced-intensity conditioning. | ||
Efficacy
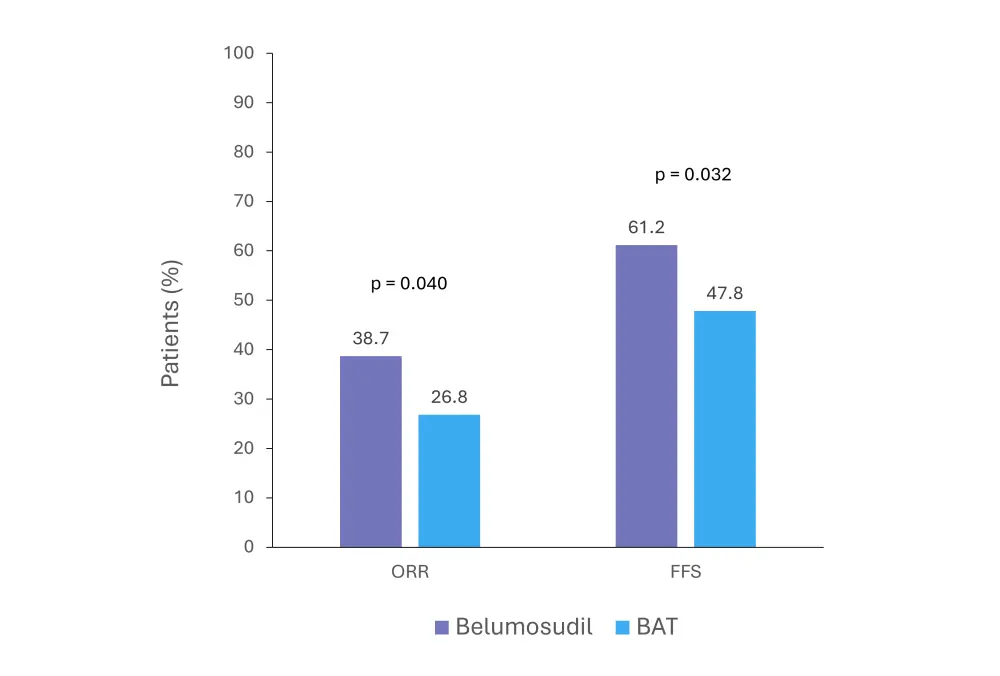
More patients treated with belumosudil achieved the primary endpoint of overall response at 6 months (Figure 2) compared with those who received BAT (38.7% vs 26.8%; p = 0.040).
Figure 2. ORR at 6 months and FFS at 1 year in the ROCKreal study*
Safety
In total, adverse events (AEs) were experienced across 27% of the belumosudil LOTs, and 36% of BAT LOTs (Table 2). The incidence of severe AEs was higher with belumosudil LOTs (58%) than for BAT LOTs (46%).
Table 2. Incidence of AEs, including severity and type, in the ROCKreal study*
| Belumosudil (LOTs N = 113) | BAT (LOTs N = 245) | ||
|---|---|---|---|---|
% | Rate per 100 LOT years | % | Rate per 100 LOT years | |
Total exposure, years | 126.3 | 341.0 | ||
Total AEs | 100.0 | 41.19 | 100.0 | 51.32 |
LOTs with ≥1 AE | 27.0 | - | 36.0 | - |
AEs due to other cGvHD therapy during study | 23.0 | 9.51 | 27.0 | 13.78 |
Patients with fatal AEs (of all patients with AEs) | 16.0 | - | 16.0 | - |
AE severity |
|
|
|
|
Moderate | 33.0 | 13.47 | 38.0 | 19.65 |
Severe | 58.0 | 23.76 | 46.0 | 23.46 |
AE type |
|
|
|
|
Infections | 37.0 | 15.05 | 40.0 | 20.53 |
Respiratory disorder | 21.0 | 8.71 | 8.0 | 4.11 |
Toxicity | 6.0 | 2.38 | 2.0 | 1.17 |
AE, adverse event; cGvHD, chronic graft-versus-host disease; LOT, line of therapy. *Adapted from Waller.2 | ||||
Conclusion
In the ROCKreal study, belumosudil showed superior efficacy compared with best available therapy, even in later LOTs. These results are consistent with those from the ROCKstar trial, providing further evidence for the safety and efficacy of belumosudil in patients with refractory cGvHD. Limitations of the study include the potential for AEs to be under-reported, and adherence to fixed monitoring schedules could also have been lacking; therefore, further real-world studies are warranted.
This educational resource is independently supported by Sanofi. All content was developed by SES in collaboration with an expert steering committee; funders were allowed no influence on the content of this resource.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
Which consideration most strongly guides your decision to escalate therapy in SR-aGvHD?





