All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional.
The gvhd Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the gvhd Hub cannot guarantee the accuracy of translated content. The gvhd and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The GvHD Hub is an independent medical education platform, sponsored by Medac and supported through grants from Sanofi and Therakos. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View GvHD content recommended for you
Prognostic significance of clinical patient characteristics and blood biomarkers in SR- and SD-aGvHD (REACH2)
Identifying biomarkers of prognostic significance in patients with acute graft-versus-host disease (aGvHD) could facilitate more effective treatment regimens.1 The phase III REACH2 trial (NCT02913261) enrolled patients aged >12 years with steroid refractory (SR) or steroid dependent (SD) aGvHD who had undergone an allogeneic hematopoietic stem cell transplant. Patients either received ruxolitinib 10 mg twice daily or the best available therapy, which was chosen by the investigator from nine options. Baseline and Day 14 models were created to identify patient characteristics and biomarkers which could influence the likelihood of response at Day 28.
GvHD, immune, and inflammatory biomarkers were identified in patients enrolled in REACH2 and measured at baseline and Day 14 to develop models for response.1
Study design1
The study design of REACH2 has been outlined in a previous article. For the exploratory analysis, a total of 29 markers/proteins were assessed at baseline and Day 14 of treatment; however, eight of these were excluded from the end analysis. These markers are shown in Figure 1, along with the measurement method.
Figure 1. Biomarkers and measurement methods in REACH2*
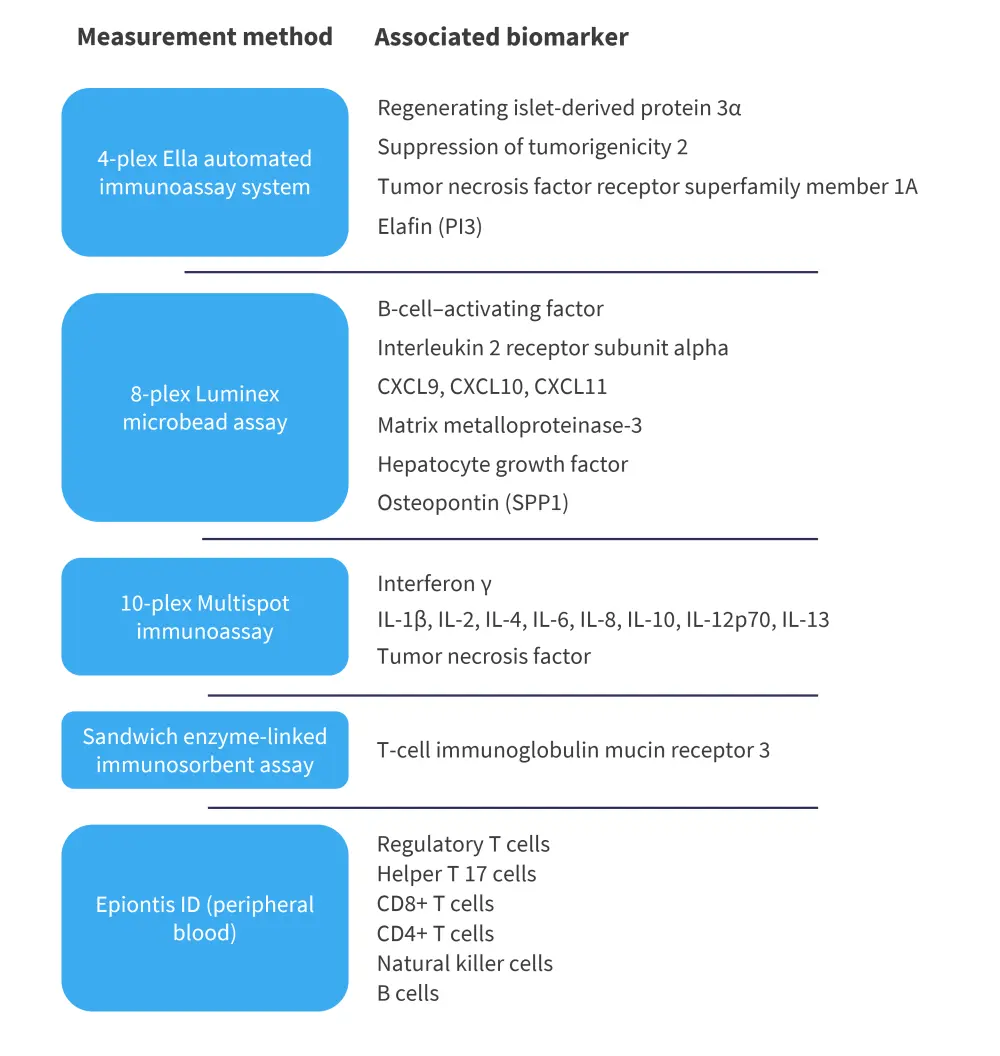
IL, interleukin.
*Adapted from Socie, et al.1
All assays were performed in multiple batches with quality control samples to ensure consistency and the batch effect was found to be acceptable on assay validation reports. In the final analysis, six biomarkers were excluded for which the baseline measurement was not within the quantification limit. Interleukin (IL) -1β, IL-12p70, IL-13, IL-2, and IL-4 were below the lower limits, and matrix metalloproteinase-3 was above the upper limits. Tumor necrosis factor and IL-10 were also excluded from additional analysis as no significant differences were found over time.
Results1
At baseline, a total of 295 patients were evaluated for presence of the remaining 21 biomarkers, with 149 of these patients receiving ruxolitinib and 146 receiving the best available therapy. Of the 295 patients, 242 had available data on at least six of the biomarkers at Day 14.
Significant biomarkers
Of the evaluated biomarkers, eight were found to vary significantly between responders and non-responders at both timepoints. These were IL-8, suppression of tumorigenicity 2, hepatocyte growth factor, regenerating islet-derived protein 3α, IL-6, IL-2Rα, tumor necrosis factor receptor superfamily member 1A, and B-cell marker. An additional four biomarkers were significantly different at Day 14: T-cell immunoglobulin mucin receptor 3, osteopontin, elafin, and CXCL10. Among all biomarkers that differed significantly, plasma concentrations were found to be higher in non-responders than responders.
Important covariates
In the baseline model, important covariates were found include patient age, conditioning regimen, and aGvHD biomarkers. Increasing age was found to decrease the probability of a clinical response. Furthermore, use of myeloablative conditioning and elevated levels of all biomarkers apart from CXCL9 and CXCL11 were also associated with a decreased probability of clinical response;
Important covariates were similar in the Day 14 model. Use of myeloablative conditioning and skin involvement at baseline decreased and increased the probability of clinical response, respectively. The biomarker value at Day 14 was also significant to response and, in both models, ruxolitinib treatment increased the probability of clinical response.
Conclusion
Overall, aGvHD biomarkers demonstrated prognostic value in both models and immune biomarkers had prognostic value in the Day 14 model.1 Certain patient characteristics were also found to have prognostic value, such as age and skin involvement. Also, treatment with ruxolitinib increased the probability of response at Day 28 compared with the best available therapy. These characteristics impacted prognostic value independently of each other and could have an additive effect. Further studies on the role of these covariates in the GvHD development may provide further insight.1
This analysis is limited by its categorization of biomarkers (aGvHD, immune, or inflammatory), as some biomarkers may have multiple functions within GvHD development. However, this categorization was necessary due to the number of baseline patient characteristics and biomarkers included.1 Further studies will be required to confirm these findings and establish how they translate to clinical practice.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content


