All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional.
The gvhd Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the gvhd Hub cannot guarantee the accuracy of translated content. The gvhd and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The GvHD Hub is an independent medical education platform, sponsored by Medac and supported through grants from Sanofi and Therakos. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View GvHD content recommended for you
As previously reported on the GvHD Hub, the initial data from the phase III REACH2 trial uncovered the potential benefit of ruxolitinib over best available treatment (BAT) for patients with steroid-refractory acute graft-versus-host disease (SR-aGvHD). As a result, the oral Janus kinases (JAK) 1 and 2 inhibitor has been approved by the U.S. Food and Drug Administration (FDA) for SR-aGvHD in adult and pediatric patients aged ≥ 12 years with SR-aGvHD.
During the 47th Annual Meeting of the European Society for Blood and Marrow Transplantation (EBMT), GvHD Hub Chair, Mohamad Mohty, Hôpital Saint-Antoine and Sorbonne University, Paris, FR, presented the updated 6-month follow-up safety and efficacy data from the REACH2 trial (Figure 1). The GvHD Hub is happy to provide a revised summary.1
Figure 1. REACH2 study design and 6-month follow-up secondary endpoints*
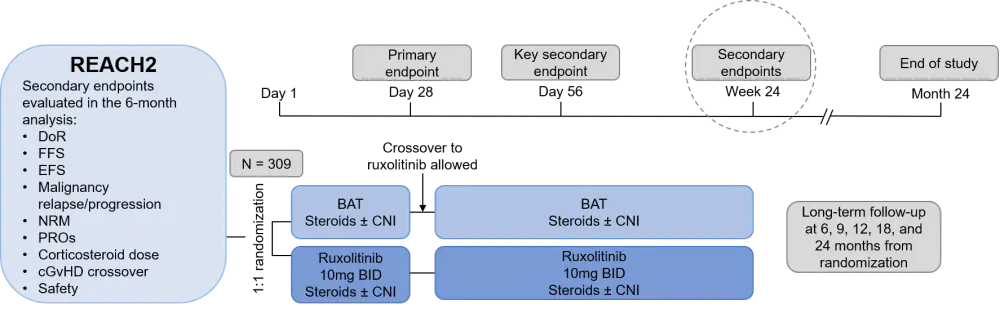
BAT, best available treatment; BID, twice daily; cGvHD, chronic graft-versus-host disease; CNI, calcineurin inhibitor; DoR, duration of response; EFS, event-free survival; FFS, failure-free survival; NRM, nonrelapse mortality; PROs, patient-reported outcome.
*Adapted from Mohty et al.1
Updated results
- Data cutoff: January 6, 2020.
- For baseline patient characteristics and results as of July 25, 2019, click here.
Efficacy
- Median duration of response, failure free-survival, and event-free survival were longer in patients who received ruxolitinib vs BAT, whereas the probability of relapse/progression and nonrelapse mortality were comparable between treatment arms (Table 1).
- EQ-5D-5L scoring was used to assess patient-reported outcomes. Improvements in mean EQ-5D-5L scores were observed across both treatment arms at week 24, which were more evident in the ruxolitinib arm.
- Of the patients in the ruxolitinib vs BAT arms
- 29.2% vs 18.7% had developed cGvHD, but fewer patients in the ruxolitinib arm developed severe cGvHD (4 vs 7).
- 22.1% vs 14.8% had completely tapered off steroids by day 56.
- Patients who crossed over to ruxolitinib at day 28 demonstrated responses comparable to those observed in the ruxolitinib arm at primary analysis.
Table 1. Outcomes of patients receiving ruxolitinib vs BAT in the REACH2 trial*
|
BAT, best available treatment; DoR, duration of response; EFS, event-free survival; FFS, failure-free survival; NRM, nonrelapse mortality. |
||||
|
Outcome |
Ruxolitinib |
BAT |
HR |
95% CI |
|---|---|---|---|---|
|
DoR, days |
163 |
101 |
|
|
|
FFS, months |
4.86 |
1.02 |
0.49 |
0.37–0.63 |
|
EFS, months |
8.18 |
4.17 |
0.80 |
0.60–1.08 |
|
6-month probability, % |
|
|
— |
— |
|
12-month probability, % NRM |
|
|
— |
— |
Safety
- No additional safety concerns were observed in the 6-month follow-up.
- In both arms, the primary cause of death was aGvHD:
- Ruxolitinib arm: 24.3%
- BAT arm: 25.3%
- Updated safety data from the 6-month follow-up are shown in Table 2.
- The incidence of serious adverse events was lower in the ruxolitinib vs BAT arm when adjusted for treatment exposure:
- Ruxolitinib arm: 319.4 per 100 patient treatment years
- BAT arm: 422.7 per 100 patient treatment years
- Cytopenia was the most commonly observed adverse event across both treatment arms.
Table 2. Safety profile of ruxolitinib vs BAT in the REACH2 trial*
|
AEs, adverse events; BAT, best available treatment. |
||
|
Safety, % |
Ruxolitinib (n = 152) |
BAT (n = 150) |
|---|---|---|
|
Grade ≥ 3 AEs |
91.4 |
87.3 |
|
Serious AEs |
66.4 |
53.3 |
|
AEs leading to discontinuation |
27.0 |
9.3 |
|
AEs leading to dose modification |
54.6 |
13.3 |
|
Deaths |
53.9 |
57.3 |
Conclusions
The 6-month follow-up of the REACH2 study highlights the sustained efficacy of ruxolitinib in patients with SR-aGvHD. Ruxolitinib demonstrated superior duration of response, failure-free survival, and event-free survival over BAT. Treatment with ruxolitinib also resulted in noticeable improvement in patient reported outcomes, and a lower probability of disease progression or the need for new systemic therapy for aGvHD. The safety profile of ruxolitinib in this setting was consistent with the primary analysis.
The GvHD Hub was happy to speak to Mohamad Mohty at the EBMT 2021, who addressed the question, What is the risk of losing response to ruxolitinib over time? You can watch the interview below.
Expert Opinion
 Mohamad Mohty
Mohamad MohtyExpert Opinion
 Robert Zeiser
Robert ZeiserFurther resources
Ruxolitinib is also being investigated for the treatment of SR-chronic GvHD in the phase III REACH3 trial, and was granted priority review by the FDA for this indication in February 2021.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

