All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional.
The gvhd Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the gvhd Hub cannot guarantee the accuracy of translated content. The gvhd and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The GvHD Hub is an independent medical education platform, sponsored by Medac and supported through grants from Sanofi and Therakos. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View GvHD content recommended for you
Novel therapies for the prevention and treatment of acute GvHD
Do you know... Which of the following agents are currently being investigated for acute GvHD prophylaxis?
Although allogeneic hematopoietic cell transplant (allo-HCT) can induce durable remissions in high-risk myeloid malignancies, acute graft-versus-host disease (aGvHD) remains a major life-threatening adverse effect after allo-HCT.1
Over the past decade, there have been major advances in the prevention and treatment of aGvHD. The GvHD Hub recently reported on repurposing drugs for GvHD prevention and treatment. Here, we summarize the recently published article by Jamy et al.1 in Blood on novel developments in the prophylaxis and treatment of aGvHD.
Novel developments in aGvHD prophylaxis
Standard GvHD prophylaxis consists of a calcineurin inhibitor, such as tacrolimus (TAC) or cyclosporine (CSA); an antimetabolite, such as methotrexate (MTX) or mycophenolate mofetil (MMF); with or without antithymocyte globulin (ATG). The choice of regimen is dependent on factors such as donor type, degree of human leukocyte antigen match, and conditioning intensity.
For 8 of 8 human leukocyte antigen-matched related and unrelated-matched donor allo-HCT with either myeloablative (MA) or reduced-intensity conditioning (RIC), prophylaxis includes MTX with either TAC or CSA. TAC/MTX has led to a lower incidence of Grade 2–4 aGvHD compared with CSA/MTX. Adding the ATG product thymoglobulin in unrelated-matched donor allo-HCT has resulted in decreased systemic immunosuppression at 12 months, with no impact on non-relapse mortality, relapse, or survival; however, data on the ATG product anti-T-lymphocyte globulin are conflicting.
For haploidentical HCT, standard prophylaxis involves posttransplant cyclophosphamide (PTCy)/TAC/MMF which has led to a lower incidence of GvHD and non-relapse mortality. Patients receiving mismatched-unrelated donor allo-HCT are at an increased risk of severe aGvHD, but prophylaxis with PTCy or abatacept has yielded promising outcomes. Recent trials investigating regimens in both RIC and MA allo-HCT have been completed.
Based on the promising data in Figure 1, many centers have now employed PTCy as a standard in RIC allo-HCT from both matched and mismatched donors, and PTCy-based regimens are commonly being used in MA allo-HCT. Moreover, abatacept is routinely used across the US in MA allo-HCT.
Figure 1. Recent completed trials for aGvHD prophylaxis*
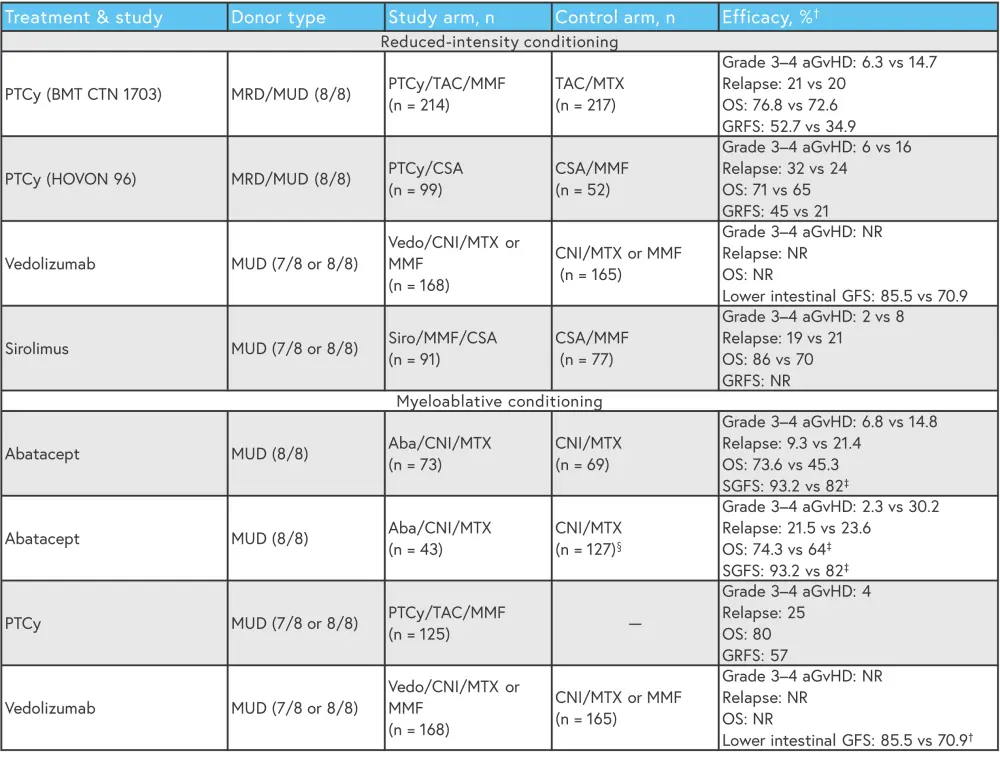
aGvHD, acute graft-versus-host disease; CNI, calcineurin inhibitor; CSA, cyclosporine; GFS, aGvHD-free survival; GRFS, GVHD/relapse or progression-free survival; MMF, mycophenolate mofetil; MTX, methotrexate; MRD, matched-related donor; MUD, matched-unrelated donor; NR, not reported; OS, overall survival; PTCy, posttransplant cyclophosphamide; SGFS, severe aGVHD-free survival; TAC, tacrolimus.
*Data from Jamy, et al.1
†Study vs control arm.
‡Statistically significant.
§CIBMTR cohort.
There are several novel regimens, such as PTCy-based combinations, JAK-inhibitor combinations, precision-engineered cell therapy (Orca-T and Orca-Q), and α1-antitrypsin (AAT), which are currently under investigation for the prevention of aGvHD (Figure 2). AAT is a serine protease inhibitor which has achieved overall response rates (ORR) and complete response (CR) rates of 65% and 35%, respectively, with a manageable safety profile in patients with steroid-refractory (SR)-aGvHD.
Figure 2. Ongoing trials for aGvHD prophylaxis*
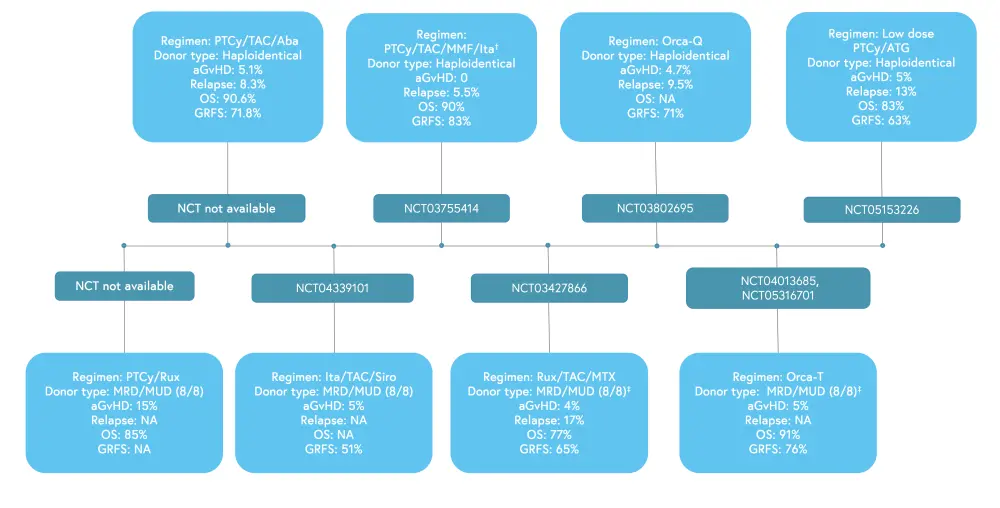
Aba, abatacept; aGvHD, acute graft-versus-host disease; ATG, antithymocyte globulin; GRFS, GVHD/relapse or progression-free survival; Ita, itacitinib; MMF, mycophenolate mofetil; MTX, methotrexate; MRD, matched-related donor; MUD, matched unrelated donor; NA, not available; OS, overall survival; PTCy, posttransplant cyclophosphamide; rux, ruxolitinib; Siro, sirolimus; TAC, tacrolimus.
*Data from Jamy, et al.1
Novel developments in the treatment of aGvHD
Despite the use of standard prophylaxis, 30–60% of patients who receive allo-HCT develop aGvHD. First-line treatment with systemic, high-dose glucocorticoids has yielded overall response rates of 50–70% and remains the standard of care for Grade ≥2 aGvHD.
Risk-stratified approaches by clinical or biomarker-based systems are likely to be adopted in future. The clinical Minnesota scoring system is commonly used; however, it identifies <15% of patients as high-risk, with no category for very low-risk patients. The MAGIC algorithm probability is a biomarker-driven strategy that can predict mortality and long-term outcomes, though it is not GvHD specific.
Outcomes are often poor for patients with Grade 3–4 aGvHD who are frequently refractory to standard glucocorticoids; even when treated with the U.S. Food and Drug Administration (FDA) approved ruxolitinib, which has achieved a higher ORR at Day 28 compared with the best available therapy; thus there is a need for further treatment in these high-risk populations. A better understanding of GvHD pathogenesis has led to further investigation in trials focused on tissue preservation, organ resilience, and microbiome-centered strategies.
For low-risk patients stratified by the Minnesota standard risk and Ann Arbor 1 criteria, itacitinib monotherapy has yielded similar outcomes with less infections compared with matched controls treated with glucocorticoids. A phase II trial is currently investigating a corticosteroid taper method guided by both clinical and MAGIC algorithm probability biomarkers (NCT05090384).
Risk-adapted trials are being increasingly used for high-risk aGvHD, combining novel agents such as itolizumab, interleukin-22, natalizumab, AAT, and receptor-interactive protein kinase 1 (RIP1) with corticosteroids (Figure 3). GDC-8264, a RIP1, plus glucocorticoids is currently being investigated in risk-stratified high-risk aGvHD (NCT05673876) and a phase I/II trial is evaluating the novel BET Inhibitor PLX51107 for SR-aGvHD (NCT04910152).
Figure 3. Preliminary results from ongoing trials for aGvHD treatment*
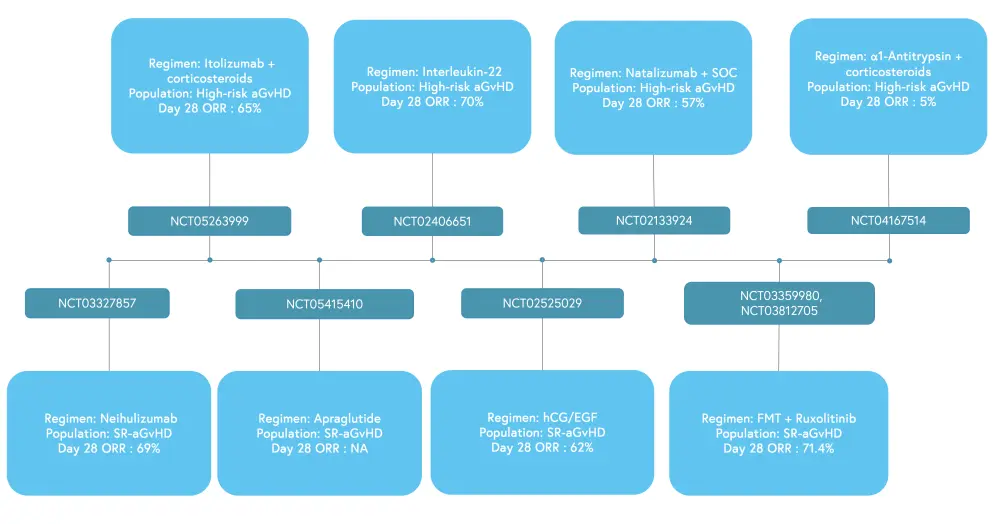
aGvHD, acute graft-versus-host disease; FMT, fetal microbiome transplantation; hCG/EGF, human chorionic gonadotropin /epidermal growth factor; NA, not available; ORR, overall response rate; SOC, standard of care; SR, steroid-refractory
*Data from Jamy, et al.1
Figure 4 provides an overview of the novel therapeutics to prevent and treat aGvHD.
Figure 4. Overview of novel therapeutics to prevent and treat aGvHD*
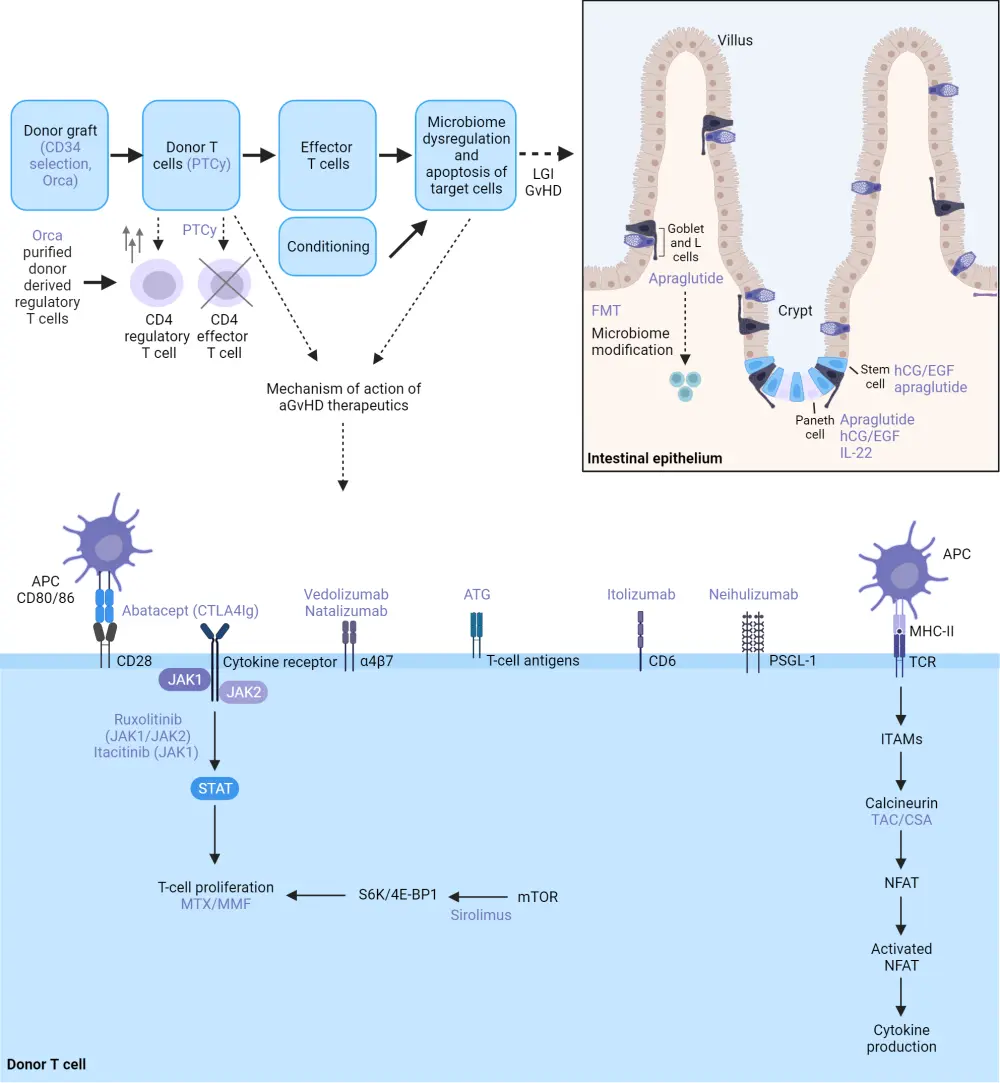
aGvHD, acute graft-versus-host disease; APC, antigen-presenting cell; ATG,antithymocyte globulin; CSA, cyclosporine; FMT, fetal microbiome transplantation; hCG/EGF, human chorionic gonadotropin/epidermal growth factor; IL-22, interleukin-22; JAK, janus kinase; LGI, lower gastrointestinal; MHC II, major histocompatibility complex II; MMF, mycophenolate mofetil; mTOR, mammalian target of rapamycin; MTX, methotrexate; NFAT, nuclear factor of activated T cells; PSGL-1, P-selectin glycoprotein ligand 1; PTCy,posttransplant cyclophosphamide; STAT, signal transducer and activator of transcription protein; TAC, tacrolimus; TCR, T-cell receptor
Adapted from Jamy, et al.1 Created with BioRender.com
Conclusion
This article highlights the significant progress made in the development of novel agents, leading to approvals and changes in the standard prophylactic treatment of aGvHD. PTCy remains the standard of care for GvHD prophylaxis in RIC allo-HCT for matched donors, and is increasingly being investigated in the matched MA setting; longer-term follow-up trials are needed to test whether the lower incidence of GvHD is associated with a survival benefit for PTCy in RIC allo-HCT.
Other novel agents being investigated for the prevention of aGvHD include combinations of abatacept, vedolizumab, ATG, and JAK inhibitors, with both PTCy and CNI/antimetabolite backbones. Longer-term trials on vedolizumab are warranted to investigate the maximum stage of gastrointestinal GVHD, its impact on skin and liver aGVHD, chronic GVHD, infectious complications, and relapse incidence.
High-dose systemic glucocorticoids remain the standard of care for high-risk aGvHD, though treatments are now shifting from immunosuppression to tissue preservation, organ resilience, and microbiome diversity. Advancements for the treatment of aGvHD include itolizumab, interleukin-22, natalizumab, AAT, and RIP1 for high-risk aGvHD and neihulizumab, apraglutide, bromodomain and extraterminal domain inhibitors, human chorionic gonadotropin/epidermal growth factor, and fecal microbiome transplants for SR-aGvHD.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
Which consideration most strongly guides your decision to escalate therapy in SR-aGvHD?


