All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional.
The gvhd Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the gvhd Hub cannot guarantee the accuracy of translated content. The gvhd and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The GvHD Hub is an independent medical education platform, sponsored by Medac and supported through grants from Sanofi and Therakos. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View GvHD content recommended for you
Repurposing drugs for GvHD prevention and treatment
Do you know... Which of the following drugs is a proteasome inhibitor, approved by the U.S. Food and Drug Administration for use in myeloma and mantle cell lymphoma?
Over the past few decades, survival following allogeneic hematopoietic stem cell transplantation (allo-HSCT) has improved due to advances in supportive care, conditioning treatments, and infection prevention.1 There have been multiple new therapies approved by the U.S. Food and Drug Administration (FDA) for the prevention and treatment of graft-versus-host disease (GvHD).1
However, some of these new drugs can be expensive, especially when used long-term. Therefore, repurposing drugs that are already proven safe for other diseases may lead to lower costs and faster clinical trial timelines. Comparison of timelines for new drug development versus drug repurposing are shown in Figure 1. Here, we discuss several FDA approved therapies for other diseases that are being investigated for use in GvHD.1
Figure 1. Timelines for drug development and repurposing*
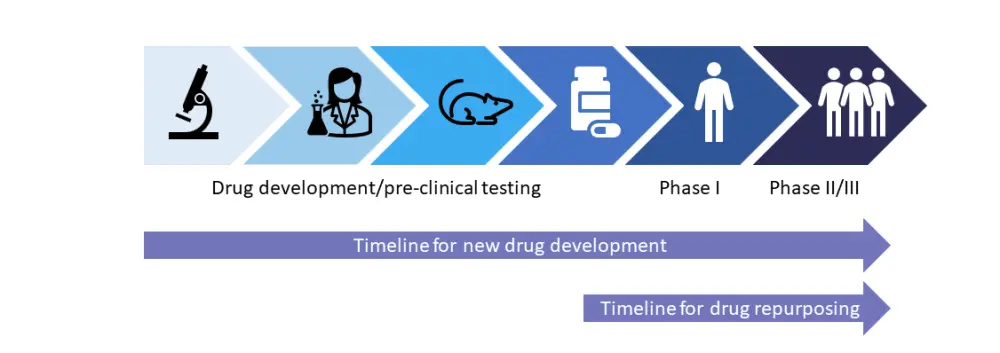
*Adapted from Farhan and Holtan.1
Overview of therapies1
Table 1. Overview of therapies being investigated for GvHD*
|
aGvHD, acute graft-versus-host disease; cGvHD, chronic graft-versus-host disease; FDA, Food and Drug Administration; GvHD, graft-versus-host disease. *Adapted from Farhan and Holtan.1 |
|||
|
Drug |
For GvHD prevention? |
For GvHD Treatment? |
Year of FDA approval for other indication |
|---|---|---|---|
|
Post-transplant cyclophosphamide |
Yes (aGvHD and cGvHD) |
No |
1999 |
|
Abatacept |
Yes (aGvHD) |
Clinical trials ongoing in cGvHD |
2005 |
|
Sitagliptin |
Yes (aGvHD) |
No |
2006 |
|
Alpha-1-antitrypsin |
Clinical trials ongoing in aGvHD and cGvHD |
No |
1987 |
|
Vitamin A |
Yes (aGvHD and cGvHD) |
No |
Vitamins not subject to approval by FDA |
|
Bortezomib |
Clinical trials ongoing in aGvHD and cGvHD |
No |
2003 |
|
Human chorionic gonadotropin |
Clinical trials ongoing in aGvHD and cGvHD |
Yes (aGvHD) Clinical trials ongoing in cGvHD |
1976 |
|
Lithium |
Clinical trials ongoing in aGvHD and cGvHD |
Clinical trials ongoing in aGvHD and cGvHD |
1970 |
Repurposed drugs for GvHD1
Post-transplant cyclophosphamide
Post-transplant cyclophosphamide (PTCy) is currently used for the prevention of acute GvHD (aGvHD) and chronic GvHD (cGvHD). PTCy initially showed positive results in the haploidentical setting, and there have been many studies since to examine PTCy in mismatched unrelated donor transplants. A prospective study by Al Malki et al. evaluated PTCy in mismatched unrelated donor blood HSCT with myeloablative and reduced intensity conditioning. At Day 100, 18% of patients had developed Grade 3–4 aGvHD, and at 1 year, 3% of patients had developed moderate-severe cGvHD. The recently reported phase III BMT 1703 trial (NCT03959241) randomized 431 patients to either tacrolimus/methotrexate (Tac/MTX) or PTCy-based prophylaxis. At one year, GvHD relapse-free survival was significantly higher in the PTCy arm (52.7% vs 34.9%) compared to the Tac/MTX arm.
One of the main risks associated with PTCy at the recommended dose of 50 mg/kg on Days 3 and 4 post-HSCT is increased infection risk. In general, PTCy costs less than other GvHD prophylaxis, but the potential for longer hospital stays due to infection and slower engraftments may lead to higher overall costs.
Antithymocyte globulin
Antithymocyte globulin (ATG) has been tested in multiple settings for GvHD prophylaxis. Meta-analyses by Kumar et al. and Yang et al. suggested that use of ATG following HSCT reduced the incidence of Grade 2–3 and Grade 3–4 aGvHD and cGvHD, with no effects on overall survival. A single-arm, phase II study individualized ATG dosing based on body weight, stem cell source, and absolute lymphocyte counts, leading to improved early CD4+ immune reconstitution in 80% of patients with no increase in GvHD.
Abatacept
Abatacept is a recombinant fusion protein first approved in 2005 by the FDA for use in rheumatoid arthritis. Clinical trials have shown that adding abatacept to GvHD prophylaxis reduces the incidence of severe aGvHD after HSCT. The ABA1 (NCT04454918) and ABA2 (NCT01743131) studies investigated abatacept for GvHD prevention. The phase II ABA2 trial in patients receiving a matched unrelated donor HSCT showed a lower rate of Grade 3–4 aGvHD in the abatacept group versus placebo (6.8% vs 14.8%, respectively). Abatacept is also being investigated as a combination therapy in an attempt to reduce the incidence of cGvHD post-HSCT. The CAST trial (NCT04503616) examining abatacept in combination with PTCy and Tac showed that the 1-year cumulative incidence of moderate-severe cGvHD was 15.9%.
Drugs currently undergoing clinical trials
Sitagliptin
Sitagliptin is a dipeptidyl peptidase-4 inhibitor approved in 2006 by the FDA for use in patients with type 2 diabetes mellitus. Dipeptidyl peptidase-4 inhibition has been shown to decrease activation of interleukin 2 and 6, but increase secretion of TGF-beta-1 in in vitro studies. In a phase II non-randomized clinical trial, in patients receiving myeloablative and reduced intensity conditioning regimens with matched related donors or unrelated donors, sitagliptin was given alongside Tac and sirolimus. At Day 100, only two of the 36 patients had developed aGvHD. A further study of sitagliptin examined its effect on the gut microbiome in patients with diabetes and in mouse models. The study found no effect on the microbiome diversity in patients with diabetes; however, the dose used in diabetes was 12 times less than that used in a clinical trial of sitagliptin for GvHD prophylaxis.
Alpha-1-antitrypsin
Alpha-1-antitrypsin (AAT), approved by the FDA for the treatment of emphysema in 1987, is currently undergoing clinical trials for autoimmune conditions such as lupus. Marcondes et al. proposed that there was an inverse correlation between the AAT plasma level in matched related donors and the risk of developing aGvHD. In murine models, mice that received cells from donors treated with AAT had a lower incidence of GvHD due to an increase in the levels of anti-inflammatory cytokines and a decrease in proinflammatory cytokines. A phase II trial suggested that AAT could be administered safely twice weekly at a dose of 60 mg/kg for up to 8 weeks, with an overall response of 65% on Day 28. This positive result has led to other prospective studies being initiated (NCT03805789 and NCT04167514).
Vitamin A
Vitamin A is implemented in numerous biological processes, including the regulation of T cell and B cell immune responses, and also affects retinoic acid signaling. Retinoic acid can enhance the conversion of naïve T cells to induced T-regulatory cells. A study at the Cincinnati Children’s Hospital showed that there was an increased incidence of gut GvHD in patients with a low plasma level of vitamin A at Day 30 after HSCT. In another study, patients with vitamin A <75th percentile of normal age were randomized either to placebo or vitamin A pre-HSCT. In the ‘as treated’ population, 10% of patients in the placebo group experienced gastrointestinal aGvHD, compared to 0% of patients in the vitamin A group.
Bortezomib
Bortezomib is a proteasome inhibitor that has been shown to have anti-apoptotic effects. It is currently approved for use in patients with myeloma and mantle cell lymphoma. The BMT CTN 1203 trial (NCT02208037) showed improved GvHD-free, relapse-free survival in the PTCy/tac/MMF arm compared with the Tac/MTX plus bortezomib or maraviroc arm. Additional studies are therefore needed to determine the optimum timing and combination for bortezomib.
Human chorionic gonadotropin
Human chorionic gonadotropin (hCG) is a hormone present during pregnancy that has been known to lead to improvement in autoimmune diseases such as Crohn’s disease and multiple sclerosis, although the mechanism for immune tolerance is unknown. In a study with patients who had cGvHD, 12 of 20 patients treated with hCG experienced substantial improvement in their cGvHD. In a study by Holtan et al., urinary-derived hCG was used instead of recombinant hCG. Urinary-derived hCG has additional growth factors such as epidermal growth factor, which is important for tissue repair. The study found that low epidermal growth factor pre-HSCT is associated with a higher risk of Grade 2–4 aGvHD by Day 100. Urinary-derived hCG and epidermal growth factors were granted Orphan Drug Designation by the FDA in 2020 for the treatment of aGvHD.
Lithium
Lithium, an inhibitor of glycogen synthase kinase-3, is involved in Wnt signaling and potentially in the differentiation of Th17. In a study, patients who received extended-release lithium carbonate tablets <7 days after salvage therapy for refractory GvHD, reported a complete response rate of 80%. When lithium was given to mice from Day -2 to Day 7 post-T-cell transplant, there was a decrease in GvHD in the ileum.
Conclusion
Prevention of GvHD can improve quality of life post-HSCT, in addition to decreasing the healthcare costs associated with HSCT.1 However, many variables can affect the efficacy of GvHD prophylactic drugs, and patients should be assessed on an individual basis.1 Further research into the pathophysiologic mechanisms that drive the disease may provide better insight into choosing first-line therapies for patients. Development of new drugs for both prevention and treatment of GvHD can take up to 10 years; therefore, repurposing old drugs with a known safety profile in humans may be a time- and cost-effective way to provide additional treatment options for patients.1
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
Which consideration most strongly guides your decision to escalate therapy in SR-aGvHD?

