All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional.
The gvhd Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the gvhd Hub cannot guarantee the accuracy of translated content. The gvhd and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The GvHD Hub is an independent medical education platform, sponsored by Medac and supported through grants from Sanofi and Therakos. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View GvHD content recommended for you
Navigating treatment sequencing of cGvHD: Real-world insights
Chronic graft-versus-host disease (cGvHD) can present with complications of variable severity affecting the skin, fascia, liver, gastrointestinal tract, eyes, mouth, and lungs.1 Corticosteroids are the standard first-line treatment to treat these complications; however, subsequent treatment options after first-line therapy are unclear, due to high levels of uncontrolled immune response and treatment-induced toxicities.1
Here, we summarize key data regarding real-world treatment sequencing at Canadian transplant centers, published by Kim et al.1 in Transplantation Proceedings.
Study design1
- A retrospective study of adult patients with cGvHD who received allogeneic hematopoietic stem cell transplantation greater than 18 months before data collection, from seven Canadian transplant centers, between September–November 2022.
- Data variables included patient characteristics, cGvHD complications information and management, and physician-reported healthcare resource utilization.
- Median values and interquartile ranges (IQR) were presented if data was not normally distributed.
Key findings1
- In total, 77 patients with a median age of 52 were included in this study.
- Overall, 99% of patients received prednisone at some point during cGvHD treatment, as shown in Table 1. The median current dose was 27.5 mg/day (IQR, 8.5–35.0).
Table 1. Treatment received by patients with GvHD*
|
ECP, extracorporeal photopheresis; FAM, fluticasone azithromycin montelukast; GvHD, graft-versus-host disease; MMF, mycophenolate mofetil; MPA, mycophenolic acid. |
|
|
Treatments ever given (may be in combination) |
All patients |
|---|---|
|
Prednisone |
76 (99%) |
|
Ruxolitinib |
41 (53%) |
|
Cyclosporine |
29 (38%) |
|
Tacrolimus |
22 (29%) |
|
Mycophenolate (as MMF or MPA) |
16 (22%) |
|
ECP |
13 (17%) |
|
Imatinib |
6 (8%) |
|
Rituximab |
5 (6%) |
|
Ibrutinib |
3 (4%) |
|
Sirolimus |
3 (4%) |
|
Other |
12 (16%) |
- First-line treatment of prednisone was paired with calcineurin inhibitors. 28% of patients received the cyclosporine combination and 16% received the tacrolimus combination.
- Ruxolitinib was the most common second-line therapy (37%), followed by mycophenolate mofetil/mycophenolic acid (17%).
- Among patients still on active systemic therapy (n = 59), prednisone and ruxolitinib were equally common at 47%.
- The most common third-line therapy was ruxolitinib (47%), again followed by mycophenolate mofetil/mycophenolic acid (17%).
- Overall, 80% of patients with cGvHD received ≤3 lines of therapy, and patients received ≥3 lines of therapy when lung complications (41%) or scleroderma (77%) were involved (Figure 1).
Figure 1. Lines of therapy received by patients with cGvHD and those with lung involvement or scleroderma*
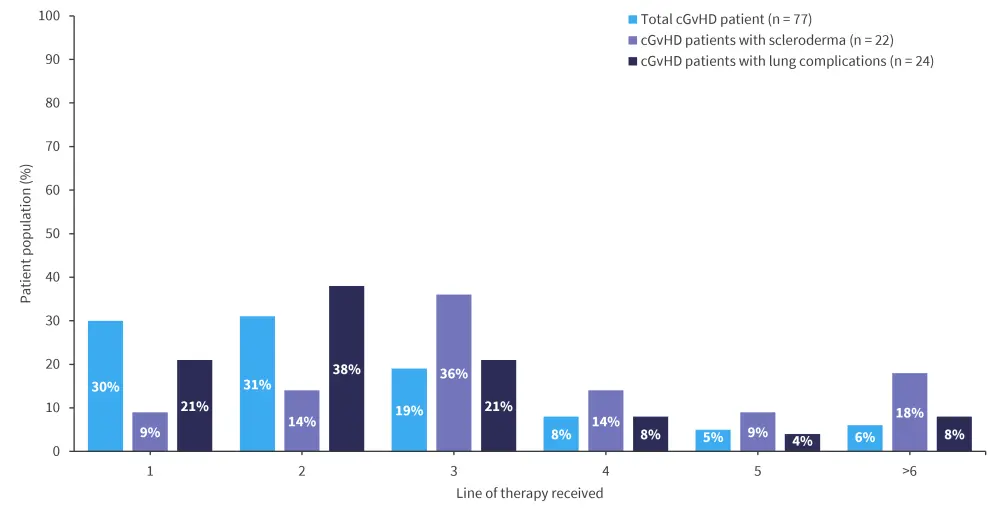
cGvHD, chronic graft-versus-host disease.
*Adapted from Dennis, et al.1
- During the management of serious complications, follow-up appointments were required more frequently than in the absence of serious complications, 14 days (IQR 7–16) and 61 days (IQR 28–84), respectively.
|
Key learnings1 |
|
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
Which consideration most strongly guides your decision to escalate therapy in SR-aGvHD?


