All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional.
The gvhd Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the gvhd Hub cannot guarantee the accuracy of translated content. The gvhd and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The GvHD Hub is an independent medical education platform, sponsored by Medac and supported through grants from Sanofi and Therakos. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View GvHD content recommended for you
Editorial theme | Defining steroid- and ruxolitinib-refractory acute and chronic GvHD
Approximately half of patients who receive an allogeneic stem cell transplant will develop graft-versus-host disease (GvHD).1 In general, aGvHD is considered to involve a skin rash, gastrointestinal symptoms, and the liver, whereas chronic GvHD (cGvHD) can involve multiple organs, including the skin, eyes, mouth, gastrointestinal tract, liver, musculoskeletal system, lung, and genitourinary system.2
Half of patients with acute GvHD (aGvHD) will not respond to corticosteroids as a first-line treatment.1 Ruxolitinib, a selective JAK 1/2 inhibitor, has been approved for use in both acute and chronic GvHD as a second-line therapy after failure with steroids.3 Therefore, it is important to define which patients are affected by steroid-refractory (SR) GvHD, to ensure appropriate treatment.
The GvHD Hub will be exploring the SR-GvHD over the next few months. For the first piece in this editorial theme, we are pleased to present a summary of the current definitions for steroid-refractoriness in patients with aGvHD and cGvHD.
Acute GvHD definitions
While defining SR-GvHD is difficult, general criteria exist to diagnose it; this is particularly useful for patients entering clinical trials. Also, reaching a consensus on the definition of SR-GvHD may ensure that comparisons across studies of SR-GvHD are more accurate.1 In 2020, Mohty et al.1 proposed definitions for SR-aGvHD and ruxolitinib-refractory aGvHD, these are outlined in Figure 1.
Figure 1. Definitions in patients with aGvHD*
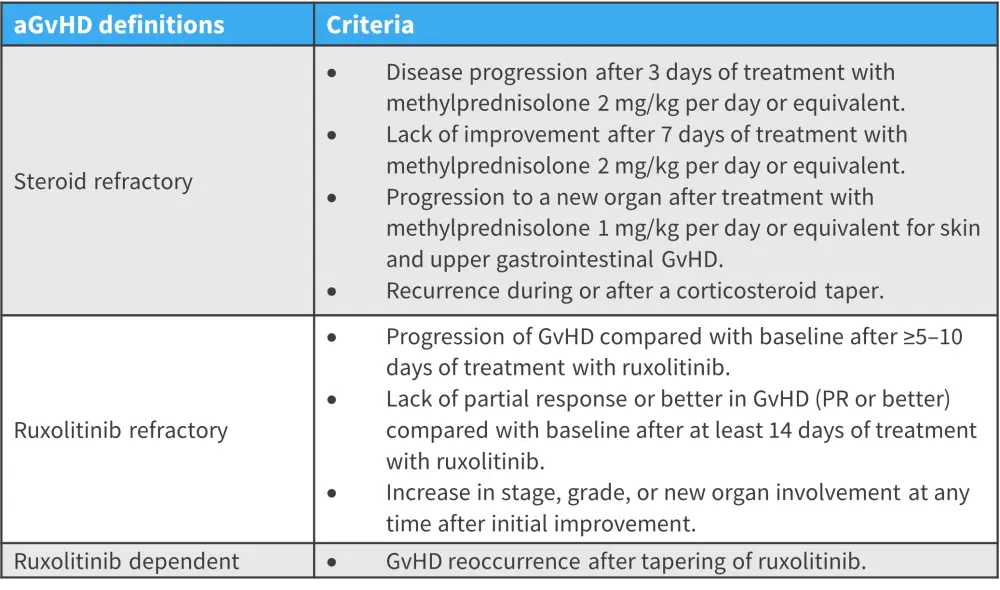
aGvHD, acute graft-versus-host disease; PR, partial response.
*Adapted from Mohty, et al.1
The definition of SR-aGvHD includes the recurrence of GvHD, which suggests there has been previous resolution. However, patients may experience a flare in GvHD symptoms following a partial response to corticosteroids. For this definition to be applied, treatment must involve an appropriate tapering of steroid doses over time; sudden decreases in dose are likely to result in a flare.1
Switching a patient from one therapy to another involves complex decision making. To optimize treatment, timely diagnosis is needed. In addition, management of corticosteroid-refractory GvHD takes into account
- GvHD severity;
- recent changes in clinical manifestations;
- current treatment; and
- tolerability of therapy.1
The severity of aGvHD is partially due to immune tolerance within individual tissues; tissues with a high degree of tolerance to immune injury can maintain health and normal function.4 However, in tissues with low tolerance, the same immune reactivity could lead to injury. In patients who are treatment refractory, immune injury may be greater than the regenerative capacity of the target organ, leading to more severe GvHD.4
Ruxolitinib-refractory GvHD1
Ruxolitinib was Food and Drug Administration (FDA) approved in 2019 for patients who have SR-aGvHD, it is now commonly used as a second-line therapy for patients who are SR. Data from REACH 1 (NCT02953678) and REACH 2 (NCT02913261) enabled ruxolitinib-refractory and ruxolitinib- dependent GvHD to be defined. Patients relapsing at the end of ruxolitinib tapering may be defined as ruxolitinib dependent. In these patients, ruxolitinib can be continued as long-term treatment is generally well tolerated.
With ruxolitinib increasingly used as a salvage therapy, it is inevitable that some patients will develop ruxolitinib-refractory GvHD. In the REACH2 trials, an overall response rate of 40% at Day 56 suggests there is a large proportion of patients who will fail to respond to ruxolitinib.
Chronic GvHD definitions2
The incidence of cGvHD has increased in the past two decades, this could be due to the increased number of older patients receiving HSCT and increase in survival rates post-HSCT. cGvHD is usually easily diagnosed in clinical practice; however, there is currently no standard clinical definition used to define refractory cGvHD.
The current definitions are shown in Figure 2, these were developed by the EMBT-NIH-CIBMTR task force. However, the current definition of SR-GvHD requires progression and clinicians may want to change treatment before progression e.g., when a patient experiences a poor or no response after a certain therapy duration. Therefore, this definition may not be suitable for clinical practice.2
Figure 2. Definitions in patients with cGvHD*
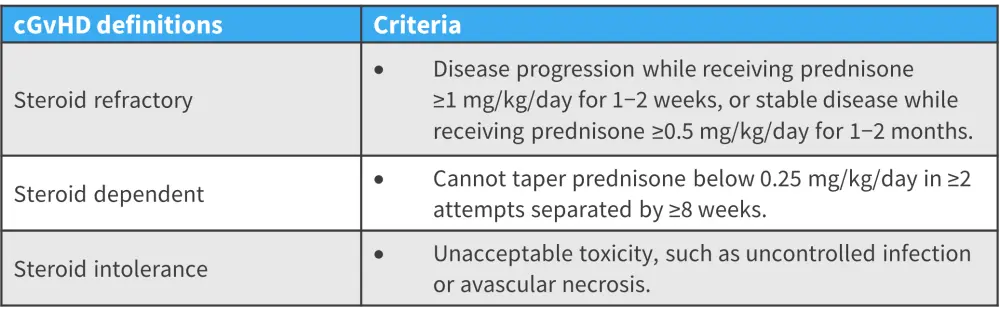
cGvHD, chronic graft-versus-host disease.
*Adapted from Pavletic.2
Biomarkers are being developed to personalize treatments for patients with cGvHD and may aid the categorization of patients as SR in the future. These biomarkers will include diagnostic, predictive, response, prognostic, and risk biomarkers; however, none are currently qualified for clinical use.
Conclusion
The grading of GvHD severity can be challenging in the real-world setting, as an improvement or decline in patient symptoms does not necessarily reflect a change in their GvHD severity. Being able to effectively diagnose SR-GvHD will ensure patients are accurately enrolled in clinical trials. However, beyond diagnosing for clinical trial enrollment, creating diagnostic criteria that consider which organs are involved for each individual means treatment approaches can be tailored, to ensure patients are switched to ruxolitinib at an appropriate time.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content




