All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional.
The gvhd Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the gvhd Hub cannot guarantee the accuracy of translated content. The gvhd and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The GvHD Hub is an independent medical education platform, sponsored by Medac and supported through grants from Sanofi and Therakos. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View GvHD content recommended for you
MaaT013, a pooled microbiota therapy, for the treatment of GI-aGvHD
At the 64th American Society of Hematology (ASH) Annual Meeting and Exposition, Mohty discussed an early-access program of MaaT013, a pooled microbiota therapy, in patients with gastrointestinal acute graft-versus-host disease (GI-aGvHD). GI-aGvHD is difficult to treat and remains an unmet medical need. Previous case reports of fecal microbiota therapy have suggested it can be safe and effective in these patients.1
Mechanism of action and study design1
The proposed mechanism of action, based on preclinical and clinical studies, is shown in Figure 1.
Figure 1. MaaT013 proposed mechanism of action*
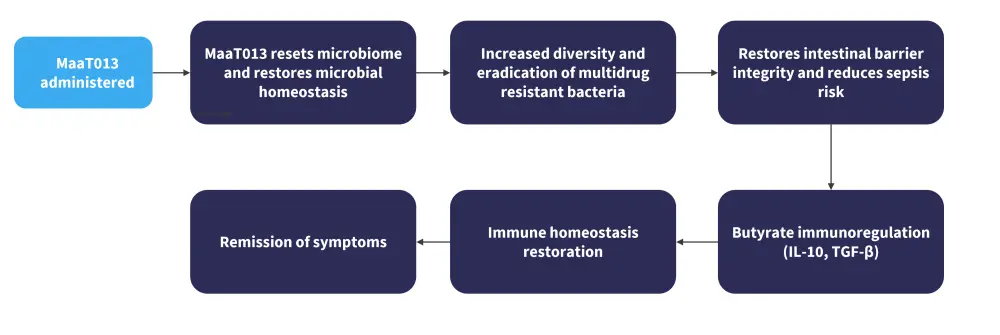
IL-10, interleukin 10; TGFβ, transforming growth factor β.
*Adapted from Mohty.1
The study enrolled 81 patients across 17 sites in France. Eligible patients were ≥18 years of age, had GI-aGvHD, and were either resistant to, or dependent on, corticosteroids with or without failure to other treatments. Patients with GvHD overlap syndrome were also accepted.
Exclusion criteria included
- active uncontrolled infection;
- a relapsed or persistent malignancy that required rapid immune suppression withdrawal;
- an uncontrolled complication; or
- evidence of toxic megacolon, bowel obstruction, or GI perforation.
Results1
Baseline patient characteristics are shown in Table 1. The majority of patients were steroid resistant, with classical type GvHD.
Table 1. Baseline patient characteristics*
|
aGvHD, acute graft-versus-host disease. |
|
|
Characteristics, % (unless otherwise stated) |
All patients (N = 81) |
|---|---|
|
Median age (range), years |
58 (18–73) |
|
Gender |
|
|
Male |
49 |
|
Female |
51 |
|
Diagnosis |
|
|
Acute myeloid leukemia |
41 |
|
Myeloproliferative syndrome |
14 |
|
Myelodysplastic syndrome |
19 |
|
Acute lymphoblastic leukemia |
14 |
|
Other |
14 |
|
Steroid status |
|
|
Resistance |
84 |
|
Dependance |
16 |
|
Median number of previous treatments for aGvHD (range), n |
2 (1–6) |
|
Median number of MaaT013 doses administered (range), n |
3 (1–3) |
GvHD-related patient characteristics shown in Figure 2.
Figure 2. A type of GvHD, B aGvHD grade at time of ATU request, and C GvHD organ involvement at inclusion*
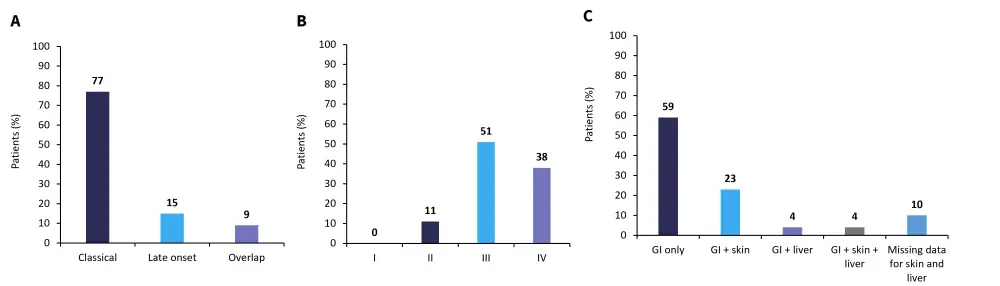
aGvHD, acute graft-versus-host-disease; ATU, temporary authorisation program; GI, gastrointestinal; GvHD, graft-versus-host disease.
*Adapted from Mohty.1
The majority of patients had Grade III or IV aGvHD (89%) at the time of request to join the program, with most patients having only GI involvement in their GvHD. All but one patient, who was administered MaaT013 by nasogastric tube, received treatment through an enema. All patients had previously received corticosteroids and 81% of patients had previously failed ruxolitinib.
Efficacy and safety
All response rates were evaluated at Day 28. Gastrointestinal-overall response rate (GI-ORR) and total ORR is shown in Figure 3A, while Figure 3B shows the GI-ORR in patients who were steroid dependant (n = 13) and steroid resistant (n = 68).
Figure 3. A ORR and GI-ORR at Day 28 and B GI-ORR in patients who were SD and SR*
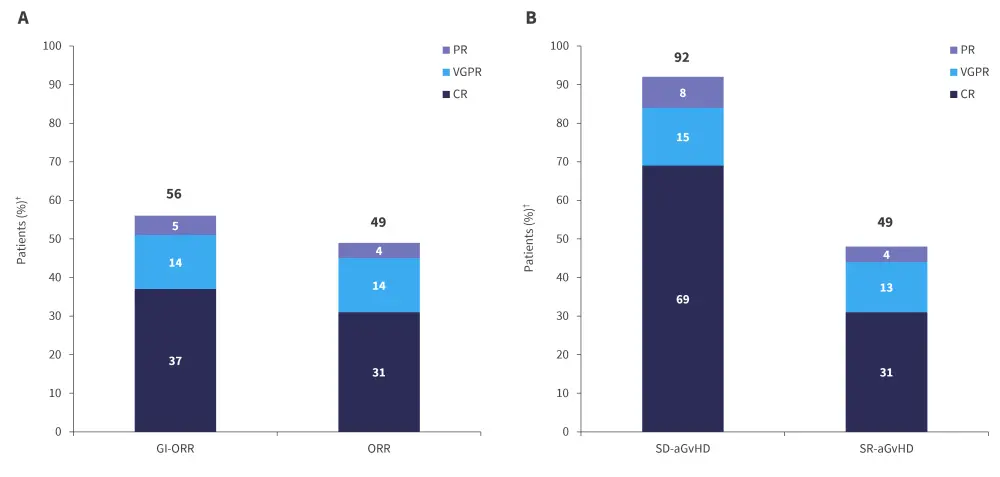
aGvHD, acute graft-versus-host disease; CR, complete response; GI-ORR, gastrointestinal overall response rate; PR, partial response; SD, steroid dependant; SR, steroid resistant; VGPR, very good partial response.
*Adapted from Mohty.1
†Missing skin and liver data at Day 28 for n = 3 patients.
The ORR of patients who were refractory to ruxolitinib at any line (n = 66) was 38%, with a GI-ORR of 56%. The GI-ORR of patients who were ruxolitinib refractory in second line and received MaaT013 as third-line therapy was 65%, with a high overall response rate of 61%.
The high overall responses and GI responses translated to significantly increased survival rates in patients, with 51% and 39% of all patients surviving to 6 and 12 months, respectively (p < 0.0001). In cases where treatment did not yield a response, 28% and 14% survived to 6 and 12 months, respectively.
In steroid and ruxolitinib refractory patients (n = 31) who were treated with MaaT-013 as a third-line therapy, 55% and 49% of patients survived to 6 and 12 months, respectively. Overall survival in patients that responded was 74% at both timepoints compared with 15% survival in non-responders at 6 months and 0% at 12 months.
There were 20 reported pharmacovigilance cases from 18 patients, with 11 cases of bacteremia or sepsis and two cases of C. difficile colitis. No instances of pathogen transmission were recorded and there were only two cases of infectious events associated with commensal bacteria.
Conclusion
This early-access program demonstrated that MaaT013 is effective for patients with GI-aGvHD, especially in patients who are steroid dependent. MaaT013 also demonstrated favorable efficacy in patients who are refractory to ruxolitinib and led to increased overall survival. No new safety signals were recorded. Further to this program, the ongoing phase III ARES study (NCT04769895) is investigating MaaT103 as a third-line agent in patients with GI-aGvHD who are steroid and ruxolitinib refractory, with a primary endpoint of GI response. The results from this study may allow MaaT013 to become approved as treatment for GI-aGvHD in the future.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
Which consideration most strongly guides your decision to escalate therapy in SR-aGvHD?



