All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional.
The gvhd Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the gvhd Hub cannot guarantee the accuracy of translated content. The gvhd and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The GvHD Hub is an independent medical education platform, sponsored by Medac and supported through grants from Sanofi and Therakos. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View GvHD content recommended for you
GvHD prophylaxis with abatacept in mismatched unrelated donor HCT
Allogeneic hematopoietic stem cell transplantation (allo-HCT) can be carried out using unrelated donors, increasing the availability of a match for patients. However, recipients of mismatched unrelated donor (MMUD) HCT are at increased risk of both acute and chronic graft-versus-host disease (GvHD), resulting in increased transplant-related mortality (TRM) and diminished overall survival (OS).1
Currently, only one agent is U.S. Food and Drug Administration (FDA) approved for the prevention of acute GvHD (aGvHD), the T-cell costimulation blockade agent abatacept in combination with a calcineurin inhibitor (CNI) and methotrexate (MTX). During the 2023 Transplantation & Cellular Therapy Meetings of ASTCT and CIBMTR, Qayed presented an analysis of abatacept for the prevention of GvHD in patients undergoing unrelated donor transplantation.1
Lessons from the ABA2 trial
Results from a phase II ABA2 study (NCT01743131), previously reported on the GvHD Hub, demonstrated positive safety and efficacy data for abatacept in combination with CNI and MTX for aGvHD, either in human leukocyte antigen (HLA) 8/8 matched unrelated (MUD) or 7/8 mismatched unrelated donor (MMUD) HCT.
To further investigate the impact of abatacept in MMUD allo-HCT, Qayed et al.1,2 compared GvHD outcomes between HLA 7/8 MMUD and 8/8 MUD cohorts in the phase II ABA2 trial. Patients in the HLA 7/8 abatacept cohort showed significant improvements in the cumulative incidence of Grade II–IV and III–IV aGvHD compared with those in the 8/8 placebo cohort. The decrease in TRM without the increase in relapse in 7/8 MMUD HCT and 8/8 MUD HCT resulted in comparable favorable survival endpoints (Figure 1). However, abatacept prophylaxis did not impact the risk of developing chronic (cGvHD).1,2
Figure 1. Comparison of GvHD and survival outcomes between HLA 7/8 and 8/8 cohorts*
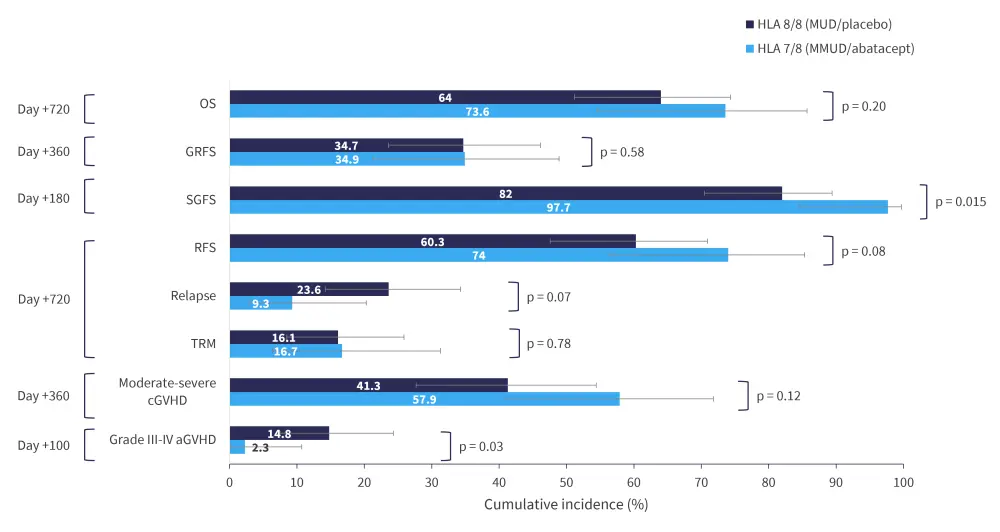
aGvHD, acute GvHD; cGvHD, chronic GvHD; GRFS, GvHD-free (absence of severe aGvHD or moderate-to-severe cGvHD) relapse-free survival; GvHD, graft-versus-host disease; HLA, human leukocyte antigen; MUD, matched unrelated donor; MMUD, mismatched unrelated donor; OS, overall survival; RFS, relapse-free survival; SGFS; severe aGVHD-free survival; TRM, transplant-related mortality.
*Adapted by Qayed, et al.2
There were no significant differences in neutrophil or platelet engraftment between the two cohorts. In MMUD/abatacept versus MUD/placebo cohorts, the Day 180 cumulative incidence of cytomegalovirus was 37.2% (95% confidence interval [CI], 24.7% to 53.4%) versus 50.5% (95% CI, 38.8% to 63.4%; p = 0.20) and Epstein-Barr virus was 46.5% (95% CI, 33.0% to 62.4%) versus 33.3% (95% CI, 22.5% to 47.5%; p = 0.14), respectively.1,2
In the HLA 8/8 group adverse event (AE) comparison analysis, patients treated with abatacept showed delayed onset of Grade II–IV, Grade III–IV, and steroid-refractory aGvHD compared with those treated with placebo (Table 1). In addition, patients treated with abatacept showed a significant decrease in Grade 3–5 AEs in the first month compared to placebo.1
Table 1. Adverse events in the HLA 8/8 group*
|
aGvHD, acute graft-versus host disease; HLA, human leukocyte antigen. *Adapted from Qayed, et al.1 |
||
|
aGvHD |
Abatacept |
Placebo |
|---|---|---|
|
Grade 0–I, n |
40 |
26 |
|
Median onset |
— |
— |
|
Grade II–IV, n |
33 |
43 |
|
Median onset |
49 (35.5–62) |
33.5 (23.8–51.3) |
|
Grade III–IV, n |
6 |
10 |
|
Median onset |
34 (19–46.5) |
30 (18.8–40.8) |
|
Steroid refractory, n |
3 |
11 |
|
Median onset |
43 (24.3–77.5) |
26 (15–38) |
Pharmacokinetic analysis and dosing
An extensive pharmacokinetics analysis by Takahashi et al.3 investigated dosing considerations for abatacept. Results showed that Ctrough_1 ≥39 μg/mL was associated with a favorable Grade II–IV aGvHD risk (hazard risk, 0.35; 95% CI, 0.19 to 0.65; p < 0.001), while Ctrough_1 <39 μg/mL was associated with a risk of Grade II–IV aGvHD that was indistinguishable from patients receiving placebo (p = 0.37). While the current abatacept dosing of 10 mg/Kg in HCT was partly derived from target steady-state Ctrough ≥10 μg/mL in rheumatoid arthritis/juvenile idiopathic arthritis cohorts, Takahashi et al.3 deduced that abatacept dose should not be capped at 1,000 mg for the prevention of GvHD.3
Real-world analysis of abatacept
Although the ABA2 study included both pediatric and adult patients, separate outcomes were not reported. Hence, Raghunandan et al.4 conducted a retrospective real-world analysis on 7/8 MMUD (n = 27) and 8/8 MUD (n = 65) pediatric patients separately, with abatacept prophylaxis for 7/8 MMUD HCT comparing favorably with the ABA2 trial, with a low incidence of Grade III-IV aGvHD and TRM while encouraging disease-free survival (DFS; Figure 2).
Raghunandan et al.5 also conducted a separate retrospective real-world analysis in adult patients ≥60 years old (n = 22) who received 7/8 MMUD HCT, revealing low rates of severe aGvHD and relapse and encouraging DFS (Figure 2).5 Overall, pediatric patients receiving 7/8 MMUD allo-HCT appeared to perform better than adults receiving 7/8 MMUD allo-HCT.1
Figure 2. Abatacept outcomes in A adult and B pediatric patients receiving unrelated donors allo-HCT*
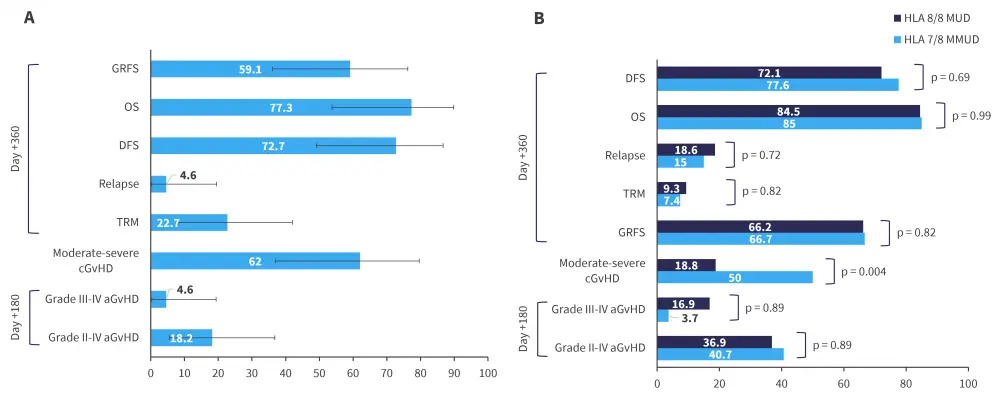
aGvHD, acute GvHD; cGvHD, chronic GvHD; DFS, disease-free survival; GRFS, GvHD-free (absence of Grade III-IV aGvHD or severe cGvHD) relapse-free survival; GvHD, graft-versus-host disease; HLA, human leukocyte antigen; MMUD, mismatched unrelated donor; MUD, matched unrelated donor; OS, overall survival; TRM, transplant-related mortality.
*Adapted by Raghunandan et al.4,5
Error bars represent 95% confidence intervals.
Overlap GvHD
Abatacept was also evaluated in overlap GvHD (defined by presence of both aGvHD and cGvHD) by Gorfinkel et al.6 in a secondary analysis of the ABA2 trial. Overlap GvHD showed more severe presentation and course than classic cGvHD within the first year post-HCT: 2-year NRM (26.1% versus 8.3%; p = 0.001), DFS (65% versus 83.2%; p = 0.02) and OS (68.5% versus 92.9%; p = 0.002). Prophylaxis with abatacept in 8/8 MUD transplantation demonstrated favorable results in NRM (overlap, 7%; classic, 10%; p = 0.89), DFS (overlap, 84%; classic, 74%; p = 0.57), or OS (overlap, 84%; classic, 89%; p = 0.59) at 2 years.6
Abatacept combinations
The combination of abatacept with posttransplant cyclophosphamide (PTCy) has been investigated in a retrospective analysis by Varshavsky-Yanovsky et al.7 Abatacept combined with PTCy appeared to be a feasible and potentially less toxic strategy for GvHD prophylaxis in 10/10 MUD and matched related donor allo-HCT.7 In a prospective single center phase Ib-II clinical trial, abatacept combined with PTCy enabled early discontinuation of tacrolimus in adult patients (n = 46), with hematological malignancies receiving related haploidentical transplantation.8
Conclusion
Data from the ABA2 trial showed that abatacept combined with CNI and MTX provides pediatric and adult patients with more donor options. Similar data from secondary analyses of ABA2 and real-world studies reinforced the advantage of abatacept as an addition to standard immunoprophylaxis in MMUD recipients. The phase II ABA3 trial (NCT04380740) is ongoing, this will further evaluate the impact of increasing abatacept doses from four to eight in patients with GvHD. Further studies are warranted to investigate the combinations with PTCy.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
Which consideration most strongly guides your decision to escalate therapy in SR-aGvHD?



