All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional.
The gvhd Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the gvhd Hub cannot guarantee the accuracy of translated content. The gvhd and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The GvHD Hub is an independent medical education platform, sponsored by Medac and supported through grants from Sanofi and Therakos. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View GvHD content recommended for you
GvHD following cord blood transplantation: guidelines for management
Acute graft-versus-host disease (aGvHD) is a noted complication of cord blood transplantation (CBT); chronic GvHD (cGvHD), while also a potential complication, occurs much less frequently following CBT compared with post-hematopoietic stem cell transplantation (HSCT) from matched related or unrelated donors. Doris M. Ponce and colleagues recently published a review outlining guidelines for the prevention and management of both aGvHD and cGvHD following CBT.1
GvHD management following CBT vs adult donor graft sources
While methotrexate (MTX) is commonly used as GvHD prophylaxis following adult donor transplantation, it should be avoided after CBT due to its negative effect on hematopoietic recovery; likewise, the utility of cyclophosphamide following transplantation remains unclear.
The management of GvHD following CBT and adult donor transplantation is largely similar, however, and the first-line treatment of aGvHD and cGvHD with corticosteroids is associated with greater responsiveness in the post-CBT setting compared with the post-adult donor transplantation setting.
Pre-engraftment syndrome vs aGvHD
Pre-engraftment syndrome (PES) is a clinical diagnosis characterized by unexplained fever and/or an erythematous rash prior to hematopoietic recovery and in the absence of documented infection; it may also be associated with renal, pulmonary, and gastrointestinal (GI) symptoms. PES is a distinct entity with an incidence of 20–87% following CBT. It occurs at a median of 7‒14 days post CBT, while the median time to aGvHD onset is 36‒40 days posttransplantation. Timing of onset is a key differentiator between PES and other posttransplantation complications, as is treatment response: most cases of PES resolve with a short course of intravenous corticosteroids, though a small number of patients may require an extended—or second—course.
It may also be helpful in differentiating between the two entities to recall that the skin is the most frequently affected organ in aGvHD; often patients have a combination of skin and GI symptoms.
GvHD prophylaxis
The most common prophylaxis regimen for GvHD is a calcineurin inhibitor (CNI) plus mycophenolate mofetil (MMF) (Table 1). Close therapeutic monitoring and dose adjustment of CNI levels early posttransplantation is important, as higher early posttransplantation CNI concentration in post-HSCT and post-CBT patients is associated with reduced risk of aGvHD. Likewise, MMF dose has been identified as a crucial factor in aGvHD risk post CBT, supporting intensified MMF dosing.
Table 1. GvHD prophylaxis following CBT*
|
CNI, calcineurin inhibitor; IV, intravenous; IVPB, intravenous piggyback; MMF, mycophenolate mofetil; PO, per oral. *Adapted from Ponce, et al.1 |
||
|
Drug |
Dose |
Therapeutic goal/comments |
|---|---|---|
|
Cyclosporine† |
Adults: 3 mg/kg IVPB every 12 h IV over 2 h starting on Day −3. |
Therapeutic range, 275–350 mg/m. |
|
Tacrolimus† |
Adults: 0.02 mg/kg/day continuous infusion IV over 24 h starting on Day −3; 0.015 mg/kg for patients aged >70 years. Actual body weight. |
Therapeutic range, 6‒12 ng/mL. The IV to PO conversion is 1:3. |
|
Mycophenolate mofetil (MMF) |
Adults: 15 mg/kg IV every 8 h starting pretransplantation; maximum 1,500 mg every 8 h. |
The IV to PO conversion is 1:1. Myfortic 180 mg is equivalent to MMF 250 mg. |
Other regimens
Less widely used regiments for GvHD prophylaxis include the following:
- Tacrolimus and sirolimus combined with antithymocyte globulin (ATG) following reduced-intensity conditioning is associated with a 9.4% cumulative incidence of Grade 2–4 aGvHD, though immune reconstitution is slow and 2-year progression-free survival (PFS) is 31%.
- Note, however, ATG is not recommended as GvHD prophylaxis for CBT due to its association with undesirable outcomes including increased transplantation-associated mortality.
- One study found tacrolimus plus sirolimus (without ATG) to be associated with a 27% rate of Grade 2–4 aGvHD and a 17% rate of Grade 3–4 aGvHD.
- Sirolimus plus MMF is a potential, but not widely used, CNI-free GvHD prophylaxis regimen.
- CNI plus MTX is commonly used in Japan, though as noted previously, MTX is associated with delayed engraftment in CBT.
Incidence of aGvHD post CBT
The reported incidence of Day 100 aGvHD in adults and children is shown in Table 2. When comparing these rates with other graft sources, additional factors such as graft type, donor-recipient human leukocyte antigen (HLA) match, and type of prophylaxis, should be considered. A lower incidence of aGvHD has been reported in patients post-CBT compared with recipients of unmodified allele-matched peripheral blood stem cell (PBSC) allografts.
Table 2. Incidence of Day 100 aGvHD*
|
*Data from Ponce, et al.1 |
||
|
Recipients |
Grade 2–4 aGvHD |
Grade 3–4 aGvHD |
|---|---|---|
|
Children |
30‒60% |
15‒30% |
|
Adults |
30‒60% |
20‒30% |
Incidence of aGvHD: single-unit vs double-unit CBT
While the data are conflicting, the consensus is that the incidence of aGvHD is increased after double-unit CBT (dCBT):
- Single-unit CBT is associated with a 20‒40% incidence of Grade 2–4 aGvHD and a 7‒10% incidence of Grade 3–4 aGvHD.
- dCBT is associated with a 30‒65% incidence of Grade 2–4 aGvHD and a 20‒35% incidence of Grade 3–4 aGvHD.
Risk factors for aGvHD
Data from early studies showed lower incidences of aGvHD when units were matched to the recipient at 6/6 HLA-A and -B antigens and -DRB1 alleles, and more recent data have shown lower incidences of Grade 2–4 aGvHD with 8/8 HLA allele matching as well as lower incidence of Grade 3–4 aGvHD after dCBT if the engrafting unit is 5‒6/6 allele matched. It should be noted, however, that the degree of allele mismatch may not predict the severity of aGvHD as other factors—including recipient age, intensity of conditioning regimen, single- or double-graft unit, use of ATG, locus-specific mismatch, and graft manipulation—may affect the incidence and severity of aGvHD.
Of these risk factors, myeloablative conditioning and absence of ATG are the most commonly cited; age ≥18 years has also been identified as a risk factor following single-unit CBT.
Treatment of post-CBT aGvHD
Patients suspected of having post-CBT aGvHD should be treated with 0.5‒2.0 mg/kg prednisone. Treatment should begin promptly, without waiting for pathologic confirmation (Figure 1).
Figure 1. Treatment of Grade 2‒4 aGvHD*
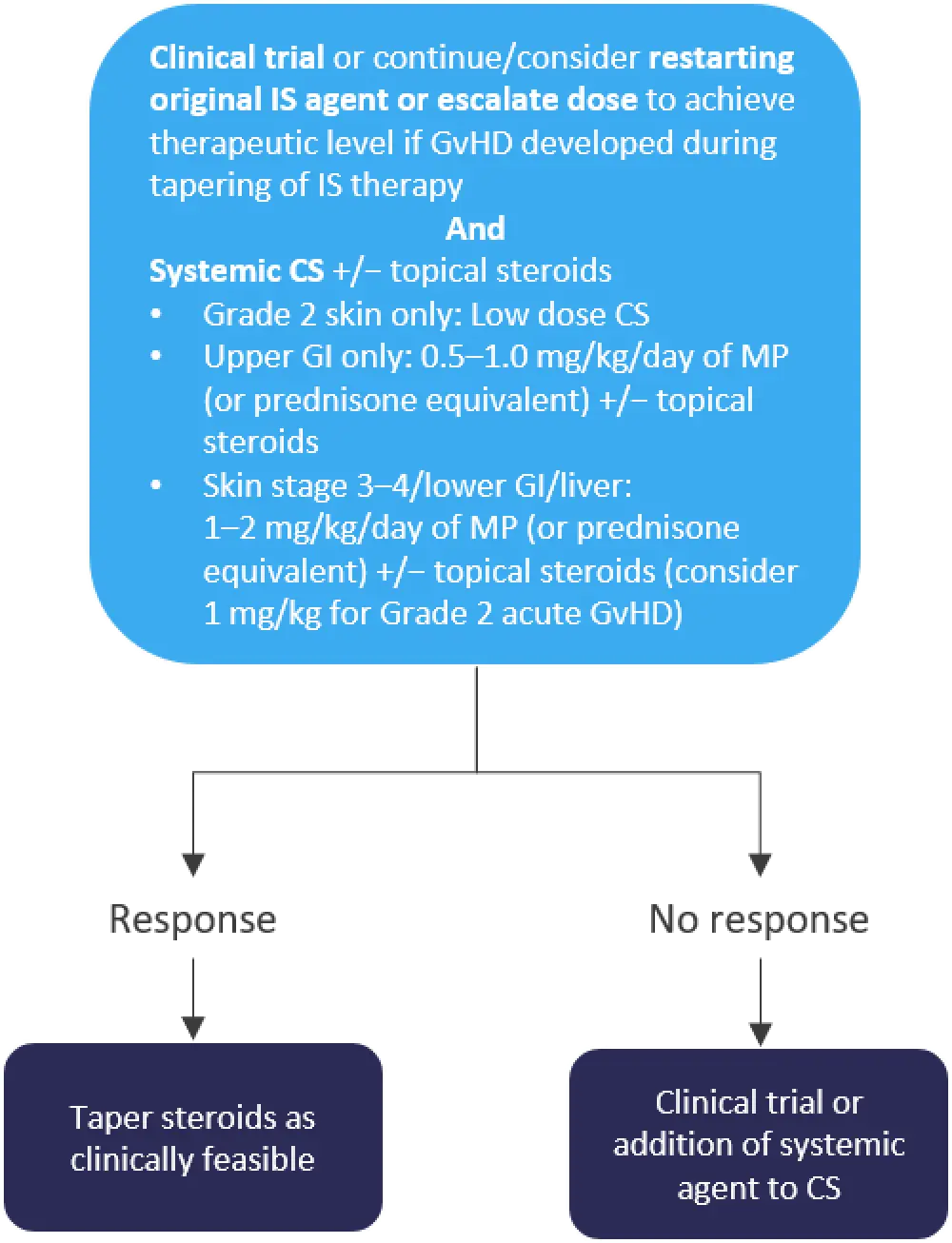
*Adapted from Ponce et al.1
CS, corticosteroids; GvHD, graft-versus-host disease; IS, immunosuppressive; MP, methylprednisolone.
Any patient with diarrhea post CB should be promptly evaluated, as lower GI aGvHD is potentially life-threatening; patients who present with non-infectious diarrhea should be treated with systemic corticosteroids, though it should be noted that Clostridioides difficile and/or viral infection can occur concurrently with aGvHD and, therefore, aGvHD should not be ruled out.
Patients can be tapered off corticosteroids once complete or partial response has been achieved, which may be as soon as 5–7 days after treatment initiation; GvHD severity and responsiveness will dictate the duration of the taper. Approximately 20% of patients, however, will not respond to up-front treatment. Patients with progression of aGvHD within 3–5 days of treatment initiation, failure to improve within 5–7 days of treatment, or incomplete response after >28 days of immunosuppressive treatment (including corticosteroids) are considered steroid-refractory or -resistant. The only U.S. Food and Drug Administration (FDA)-approved second-line treatment for these patients (adults and children ≥12 years of age) is ruxolitinib.
Post-CBT cGvHD
Post-CBT cGvHD is generally limited to mucocutaneous involvement, with the skin being the most commonly affected organ. Patients may also experience mild symptoms related to ocular and oral involvement. Severe ocular, skin, or pulmonary involvement is rare.
Incidence
Post-CBT cGvHD is generally mild to moderate and has a 2- to 3-year cumulative incidence rate of 7‒23%, with a median time to onset of 210–233 days. Classic cGvHD rarely occurs after 100 days post CBT; GvHD occurring after this time generally presents with acute manifestations as either persistent/recurrent aGvHD, late-onset aGvHD, or overlap cGvHD syndrome.
Risk factors
Patients who have previously had aGvHD are at increased risk of developing cGvHD, as are those who have received double-unit grafts.
Treatment
Upfront therapy with 0.5‒1.0 mg/kg/day of prednisone is standard treatment for moderate to severe cGvHD, while mild skin cGvHD may be treated with topical steroids. Topical steroids may also be added to systemic corticosteroids for moderate to severe GvHD. Patients who experience treatment failure on corticosteroids may be treated with ibrutinib.
Conclusion
Patients without GvHD can be tapered off immunosuppressive therapy at 3–6 months post CBT, though this practice has not been well studied, and various factors—including disease risk, toxicity, complications of immunosuppressive therapy, and degree of donor-recipient HLA match—should be considered when determining taper length and speed.
Regarding the impact of GvHD on CBT outcomes, Grade 2 aGvHD has been associated with a reduced risk of relapse, with no adverse effect on overall mortality. Grade 3–4 aGvHD has also been associated with a reduced risk of relapse, though it is also associated with increased transplantation-related mortality. cGvHD has been found to either improve or not affect survival. Overall, patients who have received CBT achieve high rates of immunosuppression discontinuation and carry low cGvHD disability-related burden, each of which contribute to improved quality of life.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
Which consideration most strongly guides your decision to escalate therapy in SR-aGvHD?

