All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional.
The gvhd Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the gvhd Hub cannot guarantee the accuracy of translated content. The gvhd and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The GvHD Hub is an independent medical education platform, sponsored by Medac and supported through grants from Sanofi and Therakos. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View GvHD content recommended for you
Fecal microbiota transplantation in high-risk lower GI acute GvHD after allo-HCT
Acute graft-versus-host disease (aGvHD) following allogeneic hematopoietic cell transplantation (allo-HCT) is often accompanied by disruption of intestinal microbiota. The GvHD Hub has previously reported on the potential of fecal microbiota transplantation (FMT) in the treatment of lower gastrointestinal (GI) aGvHD.
At the 49th Annual Meeting of the European Society for Blood and Marrow Transplantation (EBMT), DeFilipp presented phase I trial (NCT04139577) results on the feasibility and safety of third-party FMT via oral capsules for the treatment of high-risk treatment-naïve lower GI aGVHD.1 Here, we summarize the key results.
Study design1
This was an open-label, single-arm, pilot study of adult patients aged 18–75 years with high-risk aGvHD treated with frozen third-party FMT in combination with systemic corticosteroids via oral capsules. Patients with steroid-refractory or treatment-naïve (<3 days of corticosteroids ≥1 mg/kg/day) high-risk GvHD, defined by the Refined Minnesota Criteria, were included in the study. The study design is outlined in Figure 1.
Figure 1. Study design*
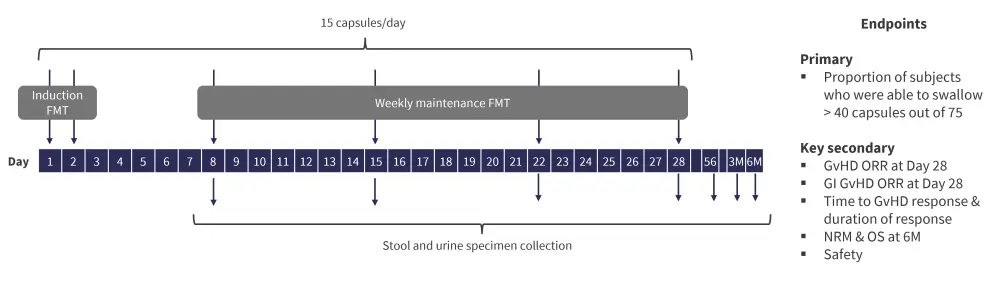
FMT, fecal microbiota transplantation; GI, gastrointestinal; GvHD, graft-versus-host disease; M, month; NRM, non-relapse mortality; ORR, overall response rate; OS, overall survival.
*Adapted from DeFilipp, et al.1
The primary objective of the study was to assess the feasibility of FMT to treat high-risk GvHD. Additionally, microbiome characterization was performed using metagenomic shotgun sequencing on donor FMT capsules and recipient stool samples. 3-indoxyl sulfate levels were measured on recipient urine samples to assess microbial diversity. The Bray distance method was used to study similarity between donor and recipient stool composition.2
Results1
A total of ten patients were enrolled in the study, with median age of 63 (range, 51–72 years). The median time from hematopoietic stem cell transplant to GvHD was 85 days. The key baseline characteristics at enrollment are shown in Figure 2.
Figure 2. Baseline patient characteristics*
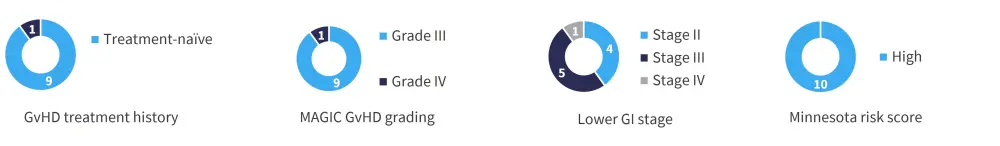
GvHD, graft-versus-host disease; MAGIC, Mount Sinai Acute GVHD International Consortium.
*Data from DeFilipp Z, et al.1
The study met its primary endpoint, with nine patients completing all eligible doses. One death was reported prior to completing the treatment due to progressive GvHD, with no treatment-related significant adverse events observed. In the first 28 days after FMT, 2 cases of bacteremia (Methicillin-resistant Staphylococcus aureus and Lactobacillus) were reported, both unrelated to FMT.
All the responders showed an initial clinical response within a week. In patients achieving complete response, clinical responses were durable and without recurrent lower GI GvHD. The median duration of response was 152 days and an ongoing GI response was observed in seven patients at data cutoff. Two deaths were reported in non-responders, both due to GvHD. The key secondary outcomes are depicted in Figure 3.
Figure 3. Key secondary endpoints: A GvHD and GI GvHD ORR at Day 28 and B NRM and OS at 6 months*
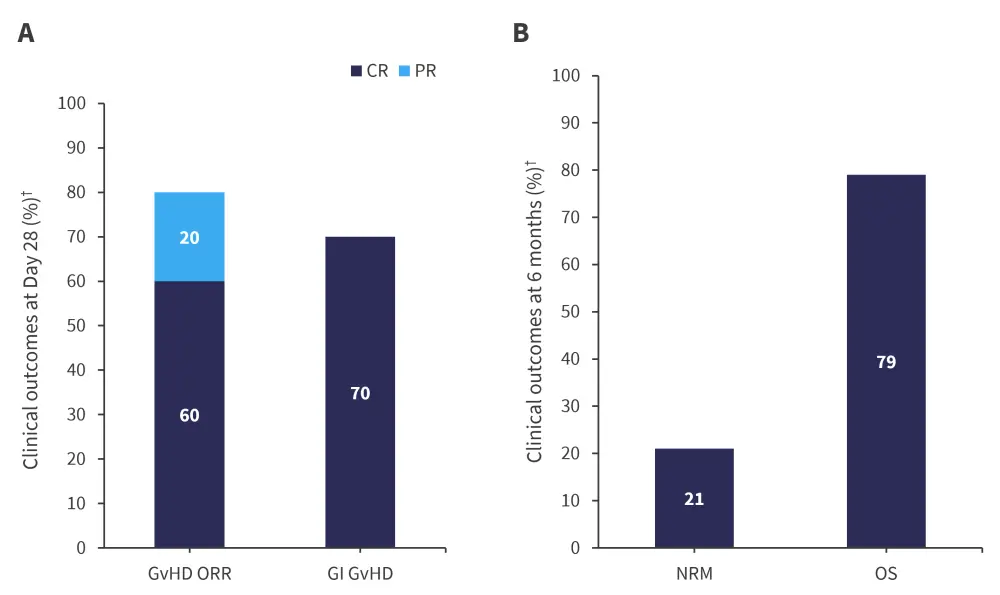
CR, complete response; GI, gastrointestinal; GvHD, graft-versus-host disease; NRM, non-relapse mortality; ORR, overall response rate; OS, overall survival; PR, partial response.
*Data from DeFilipp Z, et al.1
†Median follow-up was 311 days (range, 69–443 days).
Additionally, responders showed increased urinary 3-indoxyl sulfate at Day 28, suggesting significant improvement in microbial diversity. The Bray distance of recipient stool microbial composition trended away from baseline composition and approximating donor composition at Day 28; however, not achieving high degree of donor similarity. The metabolomics study did not show significant improvements.
Conclusion1
The authors concluded that third-party FMT is safe and feasible to be administered as oral capsules in the treatment of high-risk lower GI aGvHD. These results provide preliminary characterization of GI microbiota and metabolomics, and warrant continued investigation to optimize microbiota-based interventions in the treatment for lower GI acute GvHD.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
Which consideration most strongly guides your decision to escalate therapy in SR-aGvHD?




