All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional.
The gvhd Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the gvhd Hub cannot guarantee the accuracy of translated content. The gvhd and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The GvHD Hub is an independent medical education platform, sponsored by Medac and supported through grants from Sanofi and Therakos. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View GvHD content recommended for you
Editorial theme | T-cell depletion strategies to reduce incidence of GvHD in pediatric HSCT recipients
Do you know... Selective depletion of which donor cell type has been shown to minimize the risk of GvHD in pediatric patients following haploidentical HSCT?
The current educational theme on the GvHD Hub explores the treatment of pediatric graft-versus-host disease (GvHD). Our first two articles in this series cover consensus recommendations from the EBMT Transplant Complications Working Party and the genetic predisposition and endoscopic diagnosis of acute GvHD (aGvHD). In this third article, we explore the effect of two different T-cell depletion strategies.
While bone marrow transplantation can be of great benefit for both patients with malignant and nonmalignant hematologic disorders, the issue of finding a suitable donor remains. Haploidentical donors are accessible for most patients; however, they are associated with a high level of GvHD when the cells are given in an unmanipulated form. T-cell depletion strategies can allow haploidentical donors to be used without significant morbidity and mortality in patients. This article focuses on CD45RA+ depletion and αβ+ T-cell receptor (TCR)/CD19+ depletion in pediatric patients who have received a hematopoietic stem cell transplant (HSCT), as shown in Figure 1.
Figure 1. T-cell composition of haploidentical stem cell grafts*
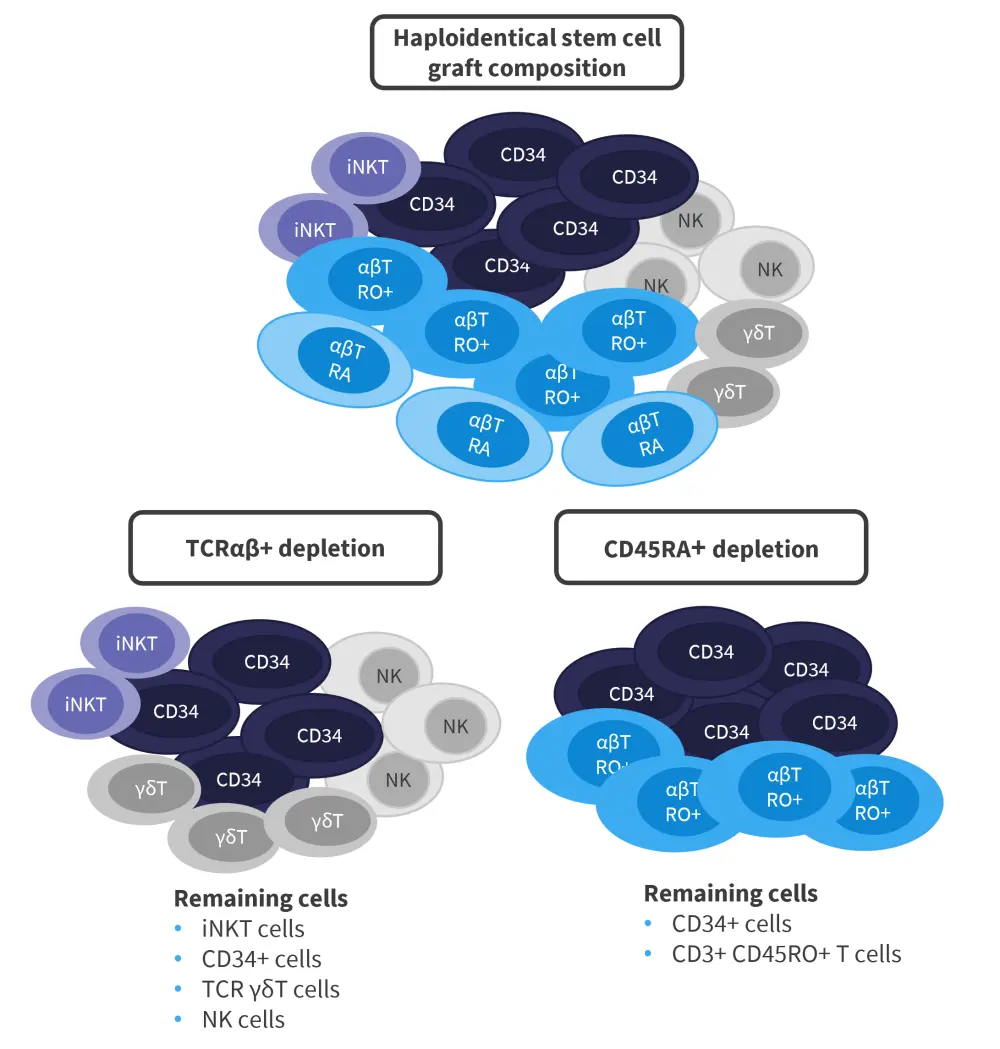
iNKT, invariant natural killer T; NK, natural killer; TCR, T-cell receptor.
*Adapted from Naik.1
CD45RA+ depletion1
CD45RA+ T cells are naïve cells that do not respond to antigen stimulation and are thought to be more alloreactive than CD45RO+ memory T cells. CD45RA+ T cells are also thought to mediate GvHD, while CD45RO+ memory T cells mediate the beneficial graft-versus-leukemia effect. By selecting the CD45RA+ T cells for depletion but allowing CD45RO+ memory T cells to remain in the graft, immune reconstitution should be promoted, while reducing the risk of disease relapse or infection. In addition, this tactic aims to minimize GvHD. Natural killer (NK) cells are partially depleted by this procedure and are added back into the graft afterwards. Viral reactivation is a concern for patients undergoing HSCT; however, maintaining CD45RO+ T cells is thought to retain specific antiviral immune activity.
During the 63rd American Society of Hematology (ASH) Annual Meeting & Exposition, Swati Naik1 presented interim data on a prospective trial (NCT01807611) in pediatric patients with high-risk hematologic malignancies treated with CD45RA+ T-cell depleted haplo-HSCT followed by NK cell addback.
The study design is shown in Figure 2 and involved a serotherapy-free, total body irradiation-free, reduced intensity conditioning regimen.
Figure 2. Study design of NCT01807611*
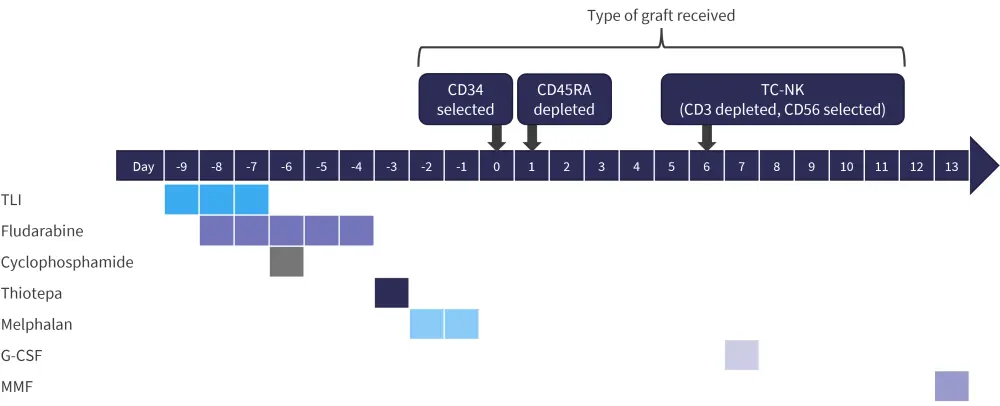
G-CSF, granulocyte-colony stimulating factor; MMF, mycophenolate mofetil; TC-NK, T cell-natural killer; TLI, T- lymphocyte irradiation.
*Adapted from Naik.1
The demographics of the patients and donors are listed in Tables 1 and 2, respectively, which show that the majority of patients were male and over half were diagnosed with acute myeloid leukemia (AML)/myelodysplastic syndrome (MDS). At the start of this trial, 26.4% of patients were classified as having refractory disease. Regarding the donors, 90.3% were parents of the recipient and 75% were mismatched at 4/8 loci.
Table 1. Patient characteristics in NCT01807611*
|
ALL, acute lymphoblastic leukemia; AML, acute myeloid leukemia; CR, complete response; MDS, myelodysplastic syndrome. |
|
|
Characteristic, % (unless otherwise stated) |
Patients (n = 72) |
|---|---|
|
Median age (range), years |
8.1 (0.6−20.8) |
|
Gender |
|
|
Male |
58.3 |
|
Female |
41.7 |
|
Diagnosis |
|
|
ALL |
40.3 |
|
AML/ MDS |
55.6 |
|
Other |
6.9 |
|
Disease status |
|
|
CR1 |
34.7 |
|
CR2 |
33.3 |
|
CR≤3 |
5.6 |
|
Refractory |
26.4 |
Table 2. Donor characteristics in NCT01807611*
|
HLA, human leukocyte antigen; MM, mismatch; NK, natural killer. |
|
|
Characteristic, % (unless otherwise stated) |
Donors (n = 72) |
|---|---|
|
Median age (range), years |
37 (18−52) |
|
Relationship |
|
|
Mother |
41.7 |
|
Father |
48.6 |
|
Sibling/other |
8.3 |
|
Degree of HLA MM |
|
|
2/8 loci |
1.4 |
|
3/8 loci |
23.6 |
|
4/8 loci |
75.0 |
|
NK alloreactive |
|
|
Yes |
86.1 |
|
No |
13.9 |
Results of the CD45RA+ depletion protocol
This method of T-cell depletion resulted in
- neutrophil engraftment in 11 days (range, 9–13 days);
- and platelet engraftment in 17 days (range 10−85 days)
Graft failure occurred in one patient but was rescued by retransplantation.
In total, 36% of patients experienced aGvHD symptoms, with 29% being classified as Grade III−IV. Of the patients that had aGvHD, 73% had gut symptoms, 54% had skin signs, and 12% showed liver symptoms.
With a median follow-up of 3.1 years (range, 0.04−8.0 years), event-free survival (EFS) was 62%. The 3-year EFS was similar for patients with acute lymphoblastic leukemia or AML/MDS. While disease status at transplant was significantly associated with EFS (p ≤ 0.0001), 3-year EFS depending on measurable residual disease status at transplant was not found to be significantly associated (p = 0.87; Table 3).
During this study, 25 patients died; 60% of these deaths were the result of progressive or relapsed disease. Non-relapse mortality occurred in ten patients, half of which had refractory disease at the time of transplant, while the other half were in complete response at transplant.
Table 3. GvHD and survival outcomes in NCT01807611*
|
ALL, acute lymphoblastic leukemia; AML, acute myeloid leukemia; CI, cumulative incidence; CR, complete response; EFS, event-free survival; GvHD, graft-versus-host disease; MDS, myelodysplastic syndrome; MRD, measurable residual disease; N/A, not available; NRM, non-relapse mortality; OS, overall survival. |
|
|
Outcome |
% |
|---|---|
|
GvHD |
|
|
CI acute GvHD |
36 |
|
Grades III–IV GvHD |
29 |
|
3-year CI of chronic GvHD |
24 |
|
Survival outcomes |
|
|
OS |
69 |
|
EFS |
62 |
|
3-year EFS for patients with ALL |
62 |
|
3-year EFS for patients with AML/MDS |
59 |
|
Disease status at transplant |
|
|
CR1 |
88 |
|
CR2 |
71 |
|
Refractory |
21 |
|
3-year EFS based on MRD status at transplant |
|
|
MRD negative |
78 |
|
MRD positive |
76 |
|
N/A |
83 |
|
Relapse and NRM |
|
|
3-year CI relapse |
26.5 |
|
3-year CI of NRM |
11.5 |
TCRαβ+/CD19+ depletion
Another T-cell depletion strategy was discussed at the 63rd ASH Annual Meeting & Exposition by Joseph Oved2 in pediatric patients with nonmalignant hematologic diseases. This strategy works on the principle that TCRαβ+/CD19+ T cells, which are thought to mediate GvHD, are depleted. CD34+ cells, which are necessary for engraftment, and TCRγδ+ T cells, which combat infection along with NK cells, are retained.3
Following previous positive results with a partial CD3+ and C19+ depletion protocol, the study discussed by Oved used a peripheral blood stem cell (PBSC) graft with TCRαβ+ and CD19+ T-cell depletion as a treatment.2 The advantages of this method are: (i) it should facilitate rapid engraftment by allowing a high dose of stem cells to be given; (ii) by partially depleting T cells, the incidence of GvHD should be reduced compared to a non-T-cell depleted unrelated donor (URD) HSCT; and (iii) there should be decreased graft rejection compared to T-cell depleted, haploidentical, or umbilical cord blood methods.2
The cohort came from a prospective clinical trial for patients with bone marrow failure (NCT03047746; n = 24) or an expanded access study (NCT03145545; n = 5). Unrelated HSCT with TCRαβ+ and CD19+ T-cell depletion of PBSCs was performed in patients with bone marrow failure, excluding those with MDS. This method retained NK cells and TCRγδ+ T cells.2
Patients were treated with the conditioning regimens shown in Figure 3, which were similar to those used in the previous study with CD3+ depletion.2
Figure 3. Conditioning regimens*
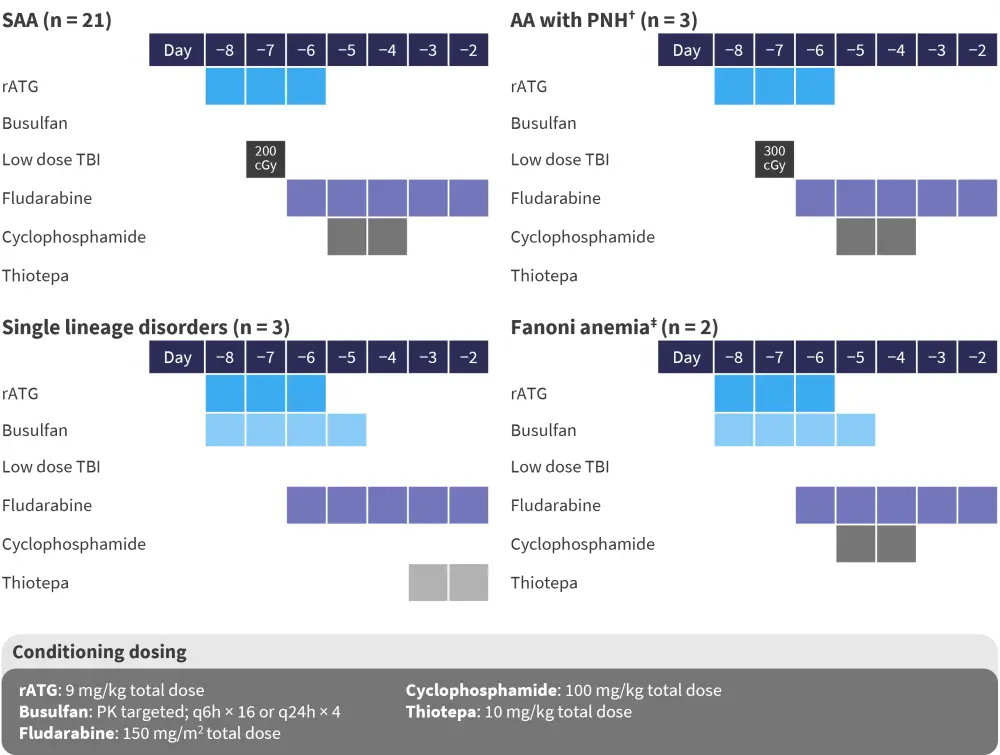
AA with PNH, aplastic anemia with paroxysmal nocturnal hemoglobinuria; rATG, rabbit anti-thymocyte globulin; SAA, severe acquired aplastic anemia; TBI, total body irradiation; TCR, T-cell receptor.
*Data from Oved.2
†Used if PNH clone >10% and in one patient on the expanded access study with a bone marrow failure syndrome of unknown etiology.
‡Dose reduction of busulfan and cyclophosphamide following Mehta, et al.4
Patient baseline characteristics for the TCRαβ+/CD19+ T-cell depletion trial are listed in Table 4. The majority of patients were non-Hispanic white, with a median age of 11 years old. Severe acquired aplastic anemia (SAA) was the most common nonmalignant hematologic disease diagnosis in this cohort. Over 50% of patients received no prior treatment for SAA.2
Table 4. Baseline characteristics for the TCRαβ+/CD19+ T-cell depletion trial*
|
BMF, bone marrow failure; CSA, cyclosporine; DBA, Diamond-Blackfan anemia; hATG, human anti-thymocyte globulin; IST, immunosuppressive therapy NOS, not otherwise specified; PNH, paroxysmal nocturnal hemoglobinuria; PRBC, peripheral red blood cell; SAA, severe acquired aplastic anemia; SCT, stem cell transplant; TCR, T-cell receptor. |
|
|
Characteristic, % (unless stated otherwise) |
n = 29 |
|---|---|
|
Age at transplant (years), median (range) |
11 (0.9−21) |
|
Female |
52 |
|
Ethnicity |
|
|
White, non-Hispanic |
62 |
|
African American |
21 |
|
Hispanic |
10 |
|
Other |
7 |
|
Disease |
|
|
SAA |
72 |
|
SAA+PNH (>10% granulocyte clone) |
10 |
|
DBA |
7 |
|
BMF NOS |
4 |
|
Fanconi anemia |
7 |
|
Median time from diagnosis to SCT (range), years |
0.4 (0.1−8.3) |
|
More than ten lifetime PRBC transfusions pre-SCT |
48 |
|
Prior SAA or SAA+PNH treatment |
|
|
IST (hATG/CSA) ×1 |
33 |
|
Multiple IST (± eltrombopag, cyclophosphamide) |
4 |
|
Eculizumab |
8 |
|
None |
54 |
Results of TCRαβ/CD19+ depletion2
Human leukocyte antigen (HLA) matching for this cohort of patients was:
- 10/10 URD, 55%;
- 9/10 URD, 41% (mismatched alleles included HLA-A, HLA-B, or HLA-DBQ1);
- and 8/10 URD, 4% (mismatches at B and DQB1).
During this study, median neutrophil and platelet engraftment both occurred at Day 15. This led to patients being discharged from hospital after 20–21 days, which was a week earlier than seen with patients treated with a matched sibling donor (MSD)-HSCT. However, it was noted that the MSD-HSCT patients received granulocyte-colony stimulating factor, unlike the TCRαβ+ depletion cohort, which may have influenced the length in hospital stay.
No cases of severe GvHD (Grade ≤III) were seen with this strategy and only three patients experienced mild GvHD that responded to steroid treatment (Table 5). There was one death recorded during the trial, leading to a disease-free survival and overall survival (OS) of 97%.
Table 5. Outcomes of the TCRαβ+/CD19+ depletion strategy*
|
cGvHD, chronic graft-versus-host disease; DFS, disease-free survival; EBV-PTLD, Epstein Barr virus-posttransplant lymphoproliferative disease; MOSF, multiple organ system failure; OS, overall survival; TA-TMA, transplant-associated thrombotic microangiopathy; TCR, T-cell receptor; VOD, veno-occlusive disease. †One death: disseminated toxoplasmosis/hemophagocytic lymphohistiocytosis leading to multi-organ system failure and death at Day 95. |
|
|
Outcome |
|
|---|---|
|
Median length of follow-up, days |
713 (42−1,581) |
|
Current DFS and OS, % |
97 |
|
Organ toxicity, n |
|
|
VOD, mild |
3 |
|
TA-TMA |
2 |
|
EBV-PTLD |
1 |
|
MOSF |
1† |
|
Mild GvHD, % |
|
|
Grade II acute |
4‡ |
|
Chronic limited (skin only) |
8§ |
TCRαβ/CD19+ depletion in the NCT01810120 trial5
In addition to the study presented by Oved, Merli et al.5 recently published an article in Blood Advances on a trial (NCT01810120) also using TCRαβ+/CD19+ depleted allogeneic-HSCT in pediatric patients with nonmalignant diseases. In this study, 70 children were enrolled, although the results from 20 of these children had been previously reported on.
In this trial, the majority of patients had severe combined immunodeficiency disorder (30%) or SAA (18.5%), and at the time of HSCT the median age was 3.6 years (range, 0.3−16.1 years). Patients received HSCT with a higher level of HLA mismatch in this trial compared with the Oved trial. The median time from diagnosis to transplant was relatively short at 10.5 months (Table 6).
Table 6. Patient, donor, and transplant characteristics in NCT01810120*
|
HLA, human leukocyte antigen; HLH, hemophagocytic lymphohistiocytosis; IBMFS, inherited bone marrow failure syndromes; PID, primary immunodeficiency; RBC, red blood cell; SAA, severe aplastic anemia; SCID, severe combined immunodeficiency; TBI, total body irradiation; thal, thalassemia. |
|
|
Characteristic, % (unless otherwise stated) |
|
|---|---|
|
Patient characteristics |
|
|
Sex |
|
|
Male |
61 |
|
Female |
39 |
|
Median age at diagnosis (range), years |
0.96 (0.0 [prenatal]–14.2) |
|
Median time from diagnosis to HSCT (range), months |
10.5 (1.2–177.7) |
|
Diagnosis |
|
|
SCID |
30 |
|
SAA |
18.5 |
|
RBC disorders |
25.5 |
|
HLH and other PID |
24 |
|
IBMFS |
4.5 |
|
Metabolic disorders |
3 |
|
Other |
7 |
|
Donor characteristics |
|
|
Median age, years (range) |
37.5 (20−51) |
|
Type of donor |
|
|
Mother |
58.5 |
|
Father |
37 |
|
Sibling |
4.5 |
|
Sex mismatch |
50 |
|
Transplant characteristics |
|
|
HLA-match |
|
|
Host-versus-graft direction |
|
|
5/10 |
60 |
|
6/10 |
28.5 |
|
7/10 |
11.5 |
|
Graft-versus-host direction |
|
|
5/10 |
46 |
|
6/10 |
31.5 |
|
7/10 |
22.5 |
|
Conditioning regimen used |
|
|
Busulfan + thiotepa + fludarabine |
34 (thal, SCID, HLH, metabolic disorders, some PIDs) |
|
Treosulfan + thiotepa + fludarabine |
26 (some PIDs, IBMFs, other) |
|
Treosulfan + fludarabine |
23 (SCID) |
|
Cyclophosphamide + fludarabine ± TBI |
15.5 (SAA) |
|
Other |
1.5 |
Results of TCRαβ/CD19+ depletion in the NCT01810120 trial
Primary donor cell engraftment was achieved in 72.8% of patients. Neutrophil and platelet recovery occurred after a median of 14.5 days (range, 9−33 days) and 10 days (range, 7−51 days), respectively.
Overall, graft failure occurred in 30.4%, with 19 cases of primary failure and two of secondary failure. Of these cases, 16 were successfully retransplanted. Sex mismatch occurred in 50% of transplants and was significantly associated with reduced incidence of graft failure. Out of the patients experiencing graft failure, three died from infectious complications.
No GvHD prophylaxis was given to patients. Despite this, the incidence of aGvHD and chronic GvHD was relatively low.
- Grade I–II skin-only aGvHD occurred in 24.2% (95% confidence interval [CI], 15.0−34.8).
- Grade II GvHD occurred in 14.4% (95% CI, 7.4−23.7).
- There was one case of mild cGvHD (in a patient with SAA who experienced primary graft failure and was given a mismatched umbilical cord blood transplant).
Overall, six patients died of infectious complications, at a median of 85 days following HSCT (range, 29−140 days). The 5-year cumulative incidence of transplant-related mortality was 8.5% (95% CI, 3.9−18.0).
For surviving patients, with a median follow-up of 3.7 years (1−9.3), the
- OS was 91.4% (95% CI, 81.9−96.1);
- EFS was 65.7% (95% CI, 53.4−75.5);
- disease-free survival was 86.8% (95% CI, 76.1−92.9);
- and GvHD-free survival was 87.1% (95% CI, 76.7−93.1).
Mixed chimerism was reported in 15.5%, 1 year after HSCT.
Conclusion
T-cell depletion strategies provide a method to increase the donor pool for transplantation by reducing the level of GvHD experienced by recipients when using non-MSDs. It may also allow for a reduced time between diagnosis and HSCT as it could enable haploidentical donors to be used who are often highly motivated to donate and available at short notice. The two different strategies used by the three groups discussed in this article did not include GvHD prophylaxis; however, the incidence of aGvHD and chronic GvHD were relatively low particularly in the TCRαβ+/CD19+ depletion studies, with mostly Grade I and II cases being recorded. In both the nonmalignant diseases and hematologic malignant disease setting, these T-cell depletion strategies provided OS levels of 69% in the CD45RA+ trial and >90% in the TCRαβ+/CD19+ trials.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
Which consideration most strongly guides your decision to escalate therapy in SR-aGvHD?



