All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional.
The gvhd Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the gvhd Hub cannot guarantee the accuracy of translated content. The gvhd and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The GvHD Hub is an independent medical education platform, sponsored by Medac and supported through grants from Sanofi and Therakos. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View GvHD content recommended for you
Editorial theme | Genetic predisposition and endoscopic diagnosis of aGvHD in pediatric patients
Do you know... The onset of Grade 3–4 aGvHD in pediatric patients has been associated with variants of which of the following genes, irrespective of non-genetic covariates?
Over the coming months, the GvHD Hub will be exploring different aspects of pediatric graft-versus-host disease (GvHD), including genetic predisposition, diagnosis, emerging therapies, and refining stem cell transplantation in this population. The first article of this editorial theme summarized consensus statements for pediatric GvHD, which you can read here. In this second article, we summarize two publications; one on the genetic susceptibility of pediatric patients to Grade 2–4 acute (a)GvHD1 and the other on the utility of upper and lower gastrointestinal (GI) endoscopy in GI aGvHD diagnosis.2
Genetic susceptibility1
The onset of aGvHD is attributed to many factors, including conditioning regimens, age, human leukocyte antigen HLA (matching), and stem cell source. Additionally, genetic variation may influence recipient susceptibility to aGvHD, independently or in association with these factors.1
An exome-wide association study, recently published by Ansari et al.1 in Bone Marrow Transplantation, investigated the association of genetic variants with the risk of Grade 2–4 aGvHD in pediatric patients.
Methods
A total of 255 patients who underwent allogeneic hematopoietic stem cell transplantation (allo-HSCT) between 2000 and 2015 were included in either a discovery or replication cohort (Figure 1).
Figure 1. Study summary*
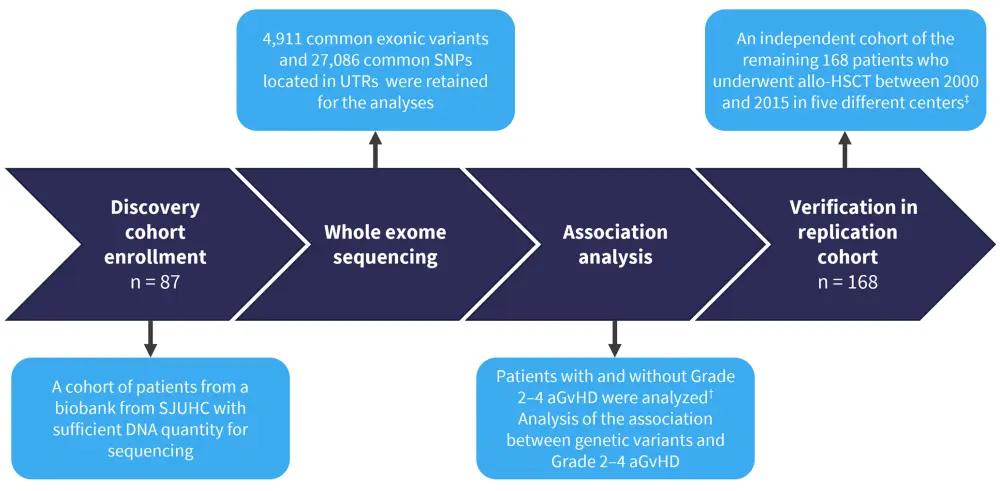
aGvHD, acute graft-versus-host disease; allo-HSCT, allogeneic hematopoietic stem cell transplantation; SJUHC, Sainte Justine University Health Center; SNP, single nucleotide polymorphism; UTR, untranslated region.
*Adapted from Ansari, et al.1
†aGvHD was diagnosed up to 180 days post allo-HSCT
‡The independent cohort comprised 47 patients from SJUHC and 121 pediatric patients who underwent allo-HSCT between 2001 and 2015 in four different centers across Europe and Canada (Geneva University Hospital, University Medical Center Utrecht, Leiden University Medical Center, Robert Debré Hospital, and Alberta Children’s Hospital).
Results
Patient characteristics for the discovery and replication cohort are summarized in Table 1.
Table 1. Patient characteristics*
|
aGvHD, acute graft-versus-host disease; HLA, human leukocyte antigen. |
||
|
Characteristics, % (unless otherwise stated) |
Discovery |
Replication |
|---|---|---|
|
Median age (range), years |
7.4 (0.1–23.5) |
4.7 (0.0–21.0) |
|
Sex |
|
|
|
Female |
54.0 |
39.9 |
|
Male |
46.0 |
60.1 |
|
Diagnosed with malignancy |
51.7 |
56.0 |
|
HLA compatibility |
|
|
|
Unrelated donor |
58.6 |
72.0 |
|
Identical related donor |
34.5 |
26.2 |
|
Non-identical related donor |
6.9 |
1.8 |
|
Stem cell source |
|
|
|
Bone marrow |
49.4 |
41.7 |
|
Peripheral blood |
2.3 |
18.4 |
|
Cord blood |
48.3 |
37.5 |
|
Bone marrow and peripheral blood |
— |
2.4 |
|
Total body irradiation |
— |
16.7 |
|
aGvHD Grade 2–4 |
12.6 |
26.8 |
|
Serotherapy |
73.6 |
73.2 |
Notable differences between the cohorts included median age, gender distribution, stem cell sources, the proportion of patients receiving total body irradiation, and Grade 2–4 aGvHD diagnosis (all p < 0.05).
Association analysis of the discovery cohort
A total of 11 loci were found to be associated with aGvHD onset after multiple testing, and nine of these were carried forward to the replication phase after correcting for multiple testing. Multivariate analysis confirmed a significant association of these variants with aGvHD onset (Table 2).
Table 2. Multivariate analysis of nine loci and Grade 2–4 aGvHD *
|
aGvHD, acute graft-versus-host disease; CI, confidence interval; HR, hazard ratio. |
||
|
Variable |
HR (95% CI) |
p value |
|---|---|---|
|
CASR rs1042636 |
31.2 (5.1–191.5) |
0.0002 |
|
SPRED1 rs11634702 |
22.7 (4.8–107.4) |
0.00008 |
|
NOP9 rs2332320 |
16.7 (3.2–86.2) |
0.001 |
|
ERC1 rs1046473 |
13.1 (3.1–55.5) |
0.001 |
|
PLEK rs3816281 |
11.4 (2.7–47.9) |
0.001 |
|
ISG20 rs59188950 |
8.4 (2.6-27.4) |
0.004 |
|
CCL8 rs1133763 |
7.4 (2.4–22.8) |
0.001 |
|
ABC11 rs17822931 |
4.7 (1.9–11.5) |
0.001 |
|
ERC1 rs1064125 |
4.6 (1.8–11.4) |
0.001 |
The investigators next analyzed whether genetic variants were impacted by other risk factors for Grade 2–4 aGvHD onset. Nongenetic covariates included patient age, type of donor, serotherapy, and stem cell source. The genetic variants found to have a significant association with aGvHD when including these nongenetic factors included ERC1, PLEK, NOP9, and SPRED1. ERC1 was associated with aGvHD in patients receiving transplants from HLA-identical siblings, as well as in bone marrow recipients and in those who did not receive serotherapy. SPRED1 was associated with aGvHD in recipients who had received serotherapy, NOP9 was associated with aGvHD in patients receiving cord blood, and PLEK was associated with aGvHD in patients without previous serotherapy (Table 3).
Table 3. Risk of aGvHD in patient subgroups with top-ranking genetic variants*
|
aGvHD, acute graft-versus-host disease; CI, confidence interval; HLA, human leukocyte antigen; HR, hazard ratio. |
|||
|
Gene |
Association group |
HR (95% CI) |
p value |
|---|---|---|---|
|
ERC1 |
Serotherapy negative patients |
3.4 (1.3–9.1) |
0.004 |
|
ERC1 |
Stem cell source: bone marrow transplant |
2.2 (1.2–4.1) |
0.007 |
|
ERC1 |
HLA-identical siblings |
2.8 (1.0–7.6) |
0.03 |
|
SPRED1 |
Serotherapy positive patients |
3.6 (1.1–12.1) |
0.02 |
|
NOP1 |
Stem cell source: cord blood |
5.8 (1.3–27.2) |
0.01 |
|
PLEK1 |
Serotherapy negative |
5.0 (0.9–28.5) |
0.04 |
Replication cohort
The nine genetic variants were tested for an association with Grade 3–4 aGvHD. Not accounting for other clinical risk factors, both ERC1 (hazard ratio, 3.3; 95% confidence interval, 1.91–9.6; p = 0.02) and PLEK (hazard ratio, 4.7; 95% confidence interval, 1.3–16.9; p = 0.003) retained significance. Serotherapy and HLA histocompatibility were also associated with Grade 3–4 aGvHD (p = 0.0002 and p = 0.01, respectively).
Diagnosis of GI aGvHD with endoscopy2
There is a lack of consensus in GI aGvHD around which GI sites to analyze, with both upper and lower GI endoscopies used for diagnosis. Comparing these sites in terms of GI GvHD sensitivity could provide insights into the optimal approach for diagnosing aGvHD.
A single centre, retrospective review of pediatric patients undergoing allo-HSCT in New Zealand was recently published by Koh et al.2 in Pediatric Transplantation, in which they reviewed the variability of endoscopic approaches used over a 12-year period. They aimed to determine which sites and methods were most useful in diagnosing GI aGvHD.
Methods
The study design is summarized in Figure 2.
Figure 2. Study design*
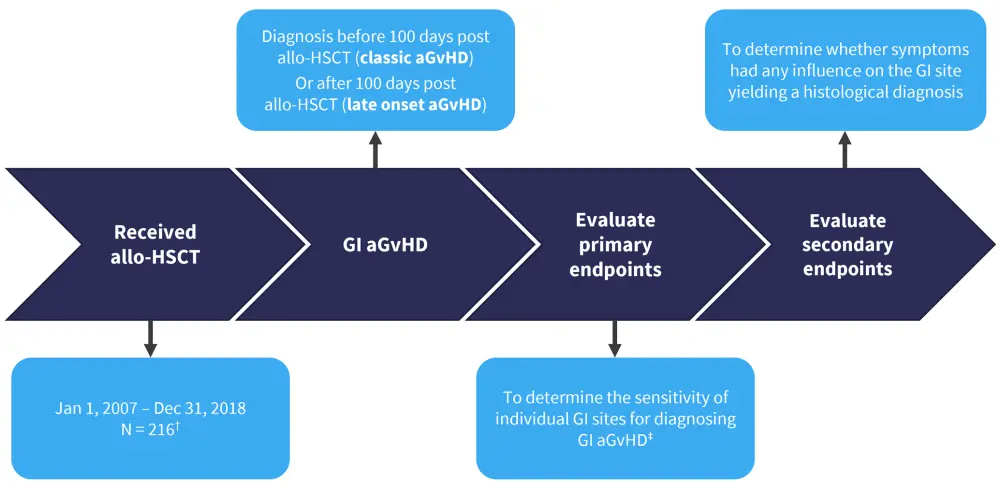
aGvHD, acute graft-versus-host disease; allo-HSCT, allogeneic hematopoietic stem cell transplantation; GI, gastrointestinal.
*Adapted from Koh, et al.2
†N = 216 refers to the total number of transplants; two separate transplants in the same patient were counted as two individual cases.
‡A positive result was defined as histologically proven GI aGvHD at the GI site. A false negative result was defined as a negative biopsy when there was histologically proven GI GvHD elsewhere in the GI tract.
Results
A total of 216 transplants were performed in 199 patients, the characteristics of which are summarized in Table 4.
Table 4. Patient and donor characteristics*
|
IQR, interquartile range. |
|
|
Characteristics |
All patients (N = 199) |
|---|---|
|
Median age at transplant (IQR), years |
6 (2–11) |
|
Sex |
|
|
Female, % |
40 |
|
Male, % |
60 |
|
Total number of transplants performed, N |
216 |
|
Related donor, n |
72 |
|
Unrelated donor, n |
144 |
A total of 52 cases of GI aGvHD were suspected, with 36 exhibiting lower GI symptoms, 5 exhibiting upper GI symptoms, and 11 cases with both. A total of 37 of these patients underwent endoscopy (Figure 3). In total, 15 patients did not receive an endoscopy: 14 patients had other non-GI sites affected by GvHD and were already receiving immunotherapy, and one patient was too unwell to receive an endoscopy.
Figure 3. Patients undergoing endoscopy and the endoscopic techniques used*
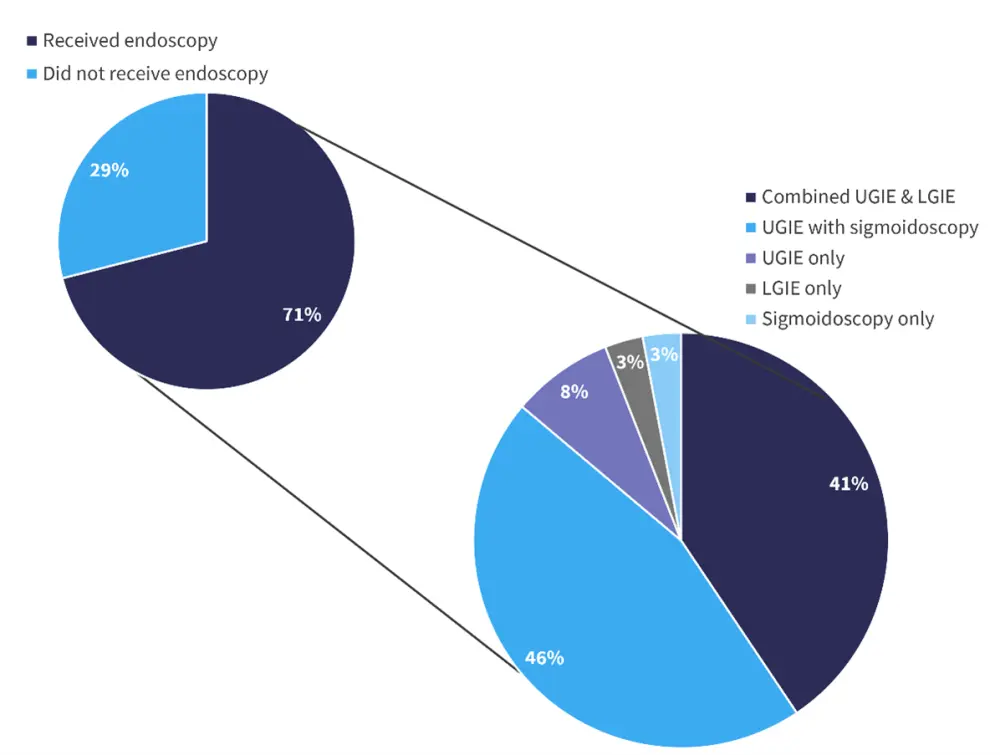
LGIE, lower gastrointestinal endoscopy; UGIE, upper gastrointestinal endoscopy.
*Adapted from Koh, et al.2
The rectosigmoid and duodenum were the two most sensitive GI regions for GvHD detection, with a sensitivity of 86% and 76%, respectively. The sensitivity of upper GI endoscopy and lower GI endoscopy for aGvHD was comparable (86% and 90%, respectively).
A total of 21 cases had histologically proven GvHD, and 19 of these patients presented with symptoms affecting non-GI sites, with 10 patients presenting with concurrent infection(s). The most common concurrent infection was cytomegalovirus, observed in seven cases.
There was no difference in GI sites affected by GvHD between late onset (>100 days) and classic aGvHD (<100 days).
Finally, there was no significant association observed between clinical symptoms and the GI site from which aGvHD was diagnosed. In 21 patients who had a positive GvHD biopsy, all had lower GI symptoms, but three of these patients did not have a positive colonic biopsy. Three out of four patients with upper GI symptoms who had an endoscopy in this site did not have a positive GI GvHD biopsy.
Conclusion
Increased risk of Grade 2–4 aGvHD in a cohort of pediatric patients was found to be associated with nine gene loci, of which the associations of four genes (ERC1, PLEK, NOP9, and SPRED1) were maintained in an interaction analysis with nongenetic covariates. Analysis for an association with Grade 3–4 aGvHD identified ERC1 and PLEK as significant independent of the nongenetic covariates studied. Study limitations included a small sample size, no genetic analysis of some donors, and a lack of an investigation into the relationship between GvHD prophylaxis and genetic variants due to heterogeneity.
In terms of diagnosis of aGvHD in pediatric patients, the retrospective study by Koh et al. demonstrated a lack of a standardized approach to endoscopy for GI aGvHD. Despite a higher occurrence of lower GI symptoms, both upper and lower GI endoscopies produced similar sensitivities for GI diagnosis. Given the lack of correlation between clinical symptoms and positive aGvHD biopsies, the authors felt that their data supported a standardized endoscopic approach utilizing upper GI endoscopy alongside sigmoidoscopy. The authors also highlighted that the study had limitations such as a small patient number, the retrospective nature of the study, and that sensitivity calculations did not account for variability in upper and lower GI endoscopic approaches.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

