All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional.
The gvhd Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the gvhd Hub cannot guarantee the accuracy of translated content. The gvhd and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The GvHD Hub is an independent medical education platform, sponsored by Medac and supported through grants from Sanofi and Therakos. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View GvHD content recommended for you
A validated clinical risk score to predict posttransplant aGvHD
Allogeneic hematopoietic cell transplantation (allo-HCT) is often followed by acute graft-versus-host disease (aGvHD) that results in significant morbidity and mortality. However, there is currently no validated clinical risk score for predicting posttransplantant aGvHD risk.1 The GvHD hub previously reported on a single-center study investigating the efficacy of a risk-scoring system of posttransplant cyclophosphamide.
At the 64th American Society of Hematology (ASH) Annual Meeting and Exposition, Ulschmid presented the first widely inclusive, validated clinical risk score to identify groups of patients with significantly different risk for aGvHD Grade II–IV and III–IV by Day 100 posttransplant. Here, we summarize the key results.1
Study design1
This analysis included patients aged ≥18 years at HCT who underwent first allo-HCT from 2008 to 2019. Patients were excluded if they had missing forms, missing consents, embargoed centers, <1 month of follow-up, rare diseases, missing disease information, mixed grafts, twin and multi-donor transplants, cord blood transplants missing units and matching information, infrequent GvHD prophylaxis regimens, missing information on aGvHD, and hyper- or sub-acute GvHD. A cohort of 21,796 donor-recipient pairs were selected and randomly split into training (n = 15,258; 70%) and validation (n = 6,538; 30%) cohorts. Patient-, disease-, and transplant-related variables were assessed at the baseline (Figure 1).
Figure 1. Variables analyzed*
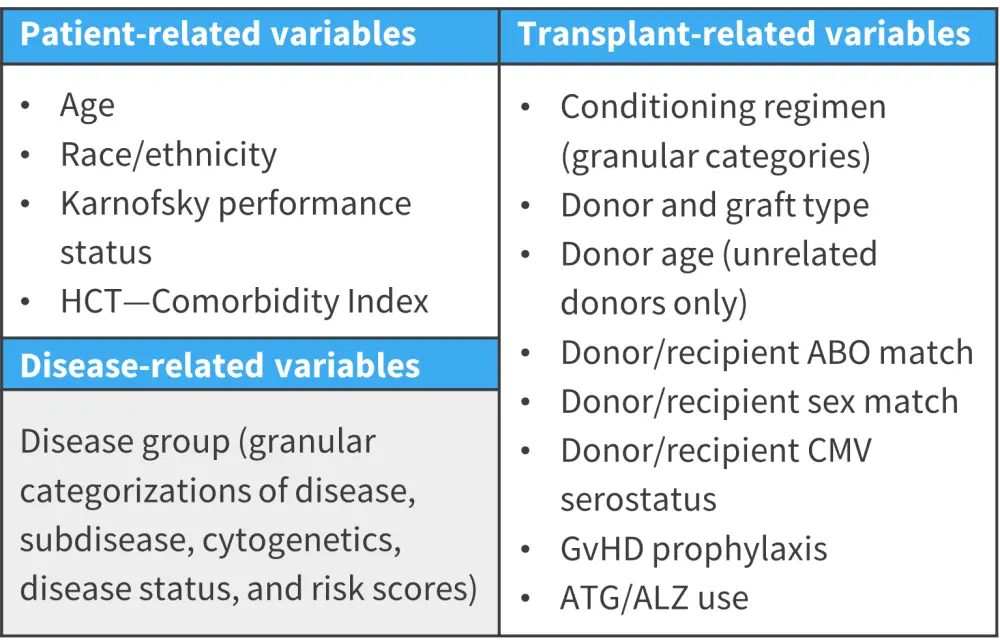
aGvHD, acute graft-versus-host disease; ALZ, alemtuzumab; ATG, anti-thrombocyte globulin; CMV, cytomegalovirus; HCT, hematopoietic cell transplantation.
*Adapted from Ulschmid, et al.1
The specific objectives were to develop and validate a clinical risk score to identify patients at significantly different risk for developing aGvHD Grades II–IV and III–IV by Day 100 posttransplant. Also, the performance of conventional logistic regression models was compared to a machine learning method known as Bayesian Additive Regression Trees (BART).
The primary outcome was Grade II–IV aGvHD and the secondary outcome was Grade III–IV aGvHD, both assessed as event rate by Day 100 posttransplant. Prognostic factors for each outcome were selected using logistic regression with a stepwise selection procedure. Based on percentile cutoffs, risk scores were categorized into four groups (≤25th percentile, 25–50th percentile, 50–75th percentile, and >75th percentile). Associations were tested using the independent validation cohort. The same training and validation cohorts were used to build and test models using BART dummy, BART factor, random forest, and extreme gradient boosting models.
Results1
The baseline patient characteristics were highly comparable in both the cohorts (Table 1).
Table 1. Key baseline patient characteristics*
|
ALL, acute lymphocytic leukemia; ALZ, alemtuzumab; AML, acute myeloid leukemia; BU, busulfan; CY, cyclophosphamide; FLU, fludarabine; HCT, hematopoietic cell transplantation; MAC, myeloablative conditioning; MDS, myelodysplastic syndrome; MEL, melphalan; MMF, mycophenolate mofetil; MTX, methotrexate; NST, non-myeloablative; PB, peripheral blood; RIC, reduced intensity conditioning; TAC, tacrolimus; TBI, total body irradiation; URD, unrelated donor. |
||
|
Characteristic, (%) unless otherwise stated |
Validation cohort |
Training cohort |
|---|---|---|
|
Median age at HCT (range), years |
55 (18–83) |
55 (18–88) |
|
Median age of the donor (URD only) (range), years |
28 (18–65) |
28 (18–68) |
|
Recipient sex |
||
|
Male |
59 |
59 |
|
Race and ethnicity |
||
|
White non-Hispanic |
72 |
72 |
|
Disease group |
||
|
AML; Intermediate cytogenetics; CR1 |
13 |
14 |
|
AML; Poor cytogenetics; CR1 |
6 |
6 |
|
Non-hodgkin lymphoma; chemosensitive |
6 |
6 |
|
MDS; IPSS-R Intermediate |
6 |
6 |
|
ALL; Poor cytogenetics; CR1 |
5 |
5 |
|
Conditioning regimen |
||
|
MAC-BU/FLU |
14 |
14 |
|
RIC-Flu/Mel ± Others |
13 |
13 |
|
RIC-BU ± Others |
12 |
12 |
|
NST-F/C/T |
12 |
12 |
|
MAC-BU/CY |
9 |
10 |
|
Donor/Graft type |
||
|
PB well matched unrelated |
33 |
32 |
|
PB HLA identical sibling |
23 |
24 |
|
Donor/recipient sex match |
||
|
M-M |
35 |
35 |
|
GvHD Prophylaxis |
||
|
TAC + MTX ± other(s) (except MMF, post-CY) |
41 |
41 |
|
TAC + MTX ± other(s) (except post-CY) |
17 |
17 |
|
Post-CY + other(s) |
16 |
16 |
|
ATG/ ALZ |
||
|
No ATG or ALZ |
74 |
74 |
From logistic regression, the odds of developing Grade II–IV aGvHD and Grade III–IV aGvHD by Day 100 posttransplant in the 25–50th, 50–75th, and >75th percentile groups were significantly higher than the ≤25th percentile groups in the validation cohort (all p < 0.0001; Figure 2).
The adjusted Day-100 probability of Grade II–IV aGvHD was 26% and 52% in the ≤25th percentile group and >75th percentile group, respectively. Likewise, adjusted Day-100 probability of Grade III–IV aGvHD was 9% and 24% in the ≤25th percentile group and >75th percentile group, respectively.
Figure 2. Odds of developing A Grade II–IV aGvHD and B Grade III–IV aGvHD by Day 100 posttransplant, as per the logistic regression model*
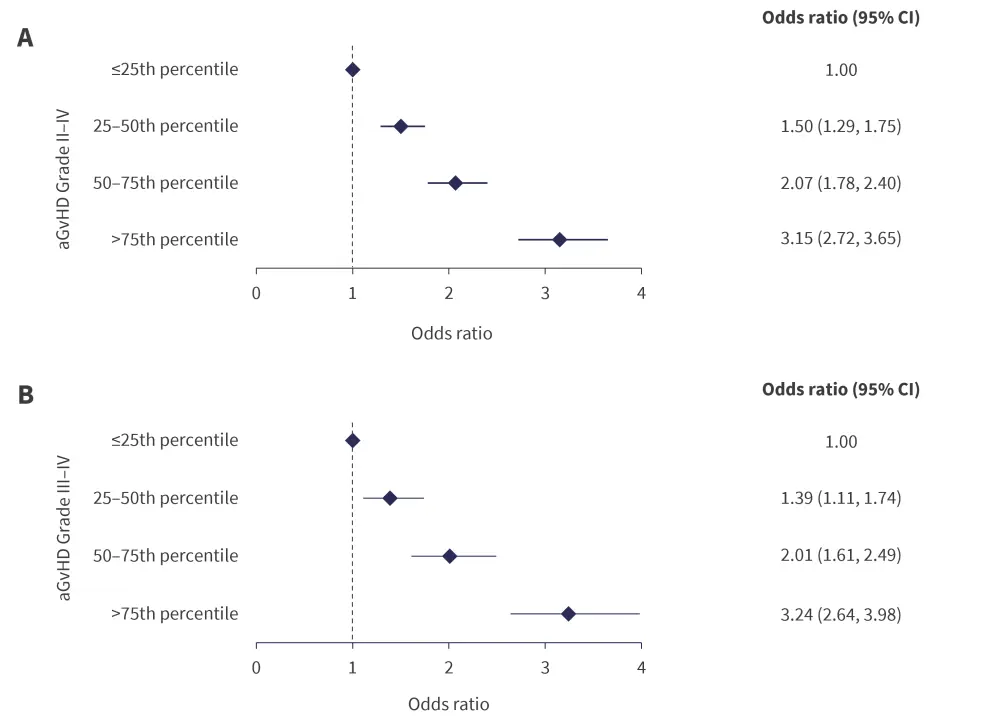
aGvHD, acute graft versus host disease; CI, confidence interval.
*Adapted from Ulschmid, et al.1
Overall, the performance of BART-based machine learning models was shown to be similar to logistic regression models when compared using concordance index (Figure 3).
Figure 3. Concordance-statistics from association tests in validation cohort*
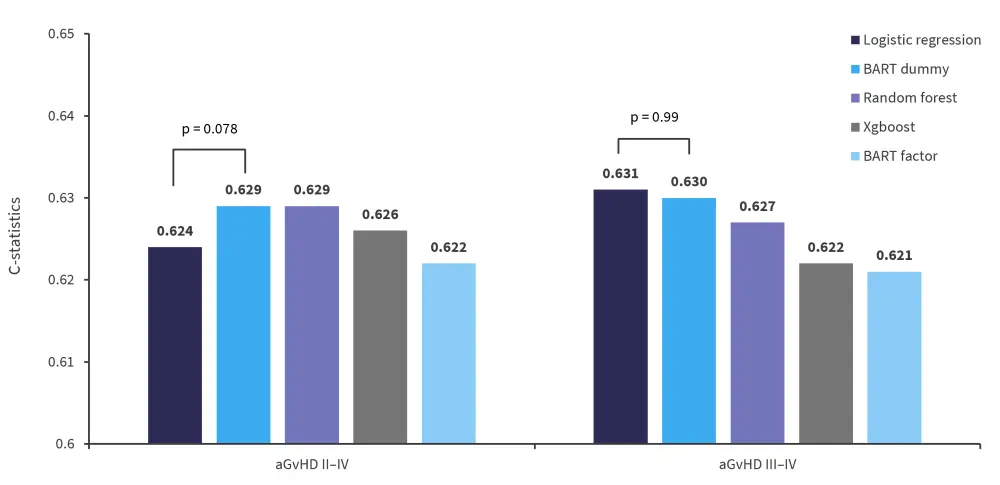
aGvHD, acute-graft-versus-host disease; BART, Bayesian additive regression trees; XGboost, extreme gradient boost.
*Adapted from Ulschmid, et al.1
Conclusion1
A validated clinical risk score for predicting the risk of developing Grade II–IV and III–IV aGvHD posttransplant was developed with wide eligibility criteria and inclusiveness. Furthermore, the performance of the BART-based machine learning models was comparable to traditional statistical-based models. These scores can aid in patient counseling, personalized clinical decision-making, and designing clinical trials. The Center for International Blood and Marrow Transplant Research (CIBMTR) plans to use this model to create an online risk score calculator.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
Which consideration most strongly guides your decision to escalate therapy in SR-aGvHD?


