All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional.
The gvhd Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the gvhd Hub cannot guarantee the accuracy of translated content. The gvhd and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The GvHD Hub is an independent medical education platform, sponsored by Medac and supported through grants from Sanofi and Therakos. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View GvHD content recommended for you
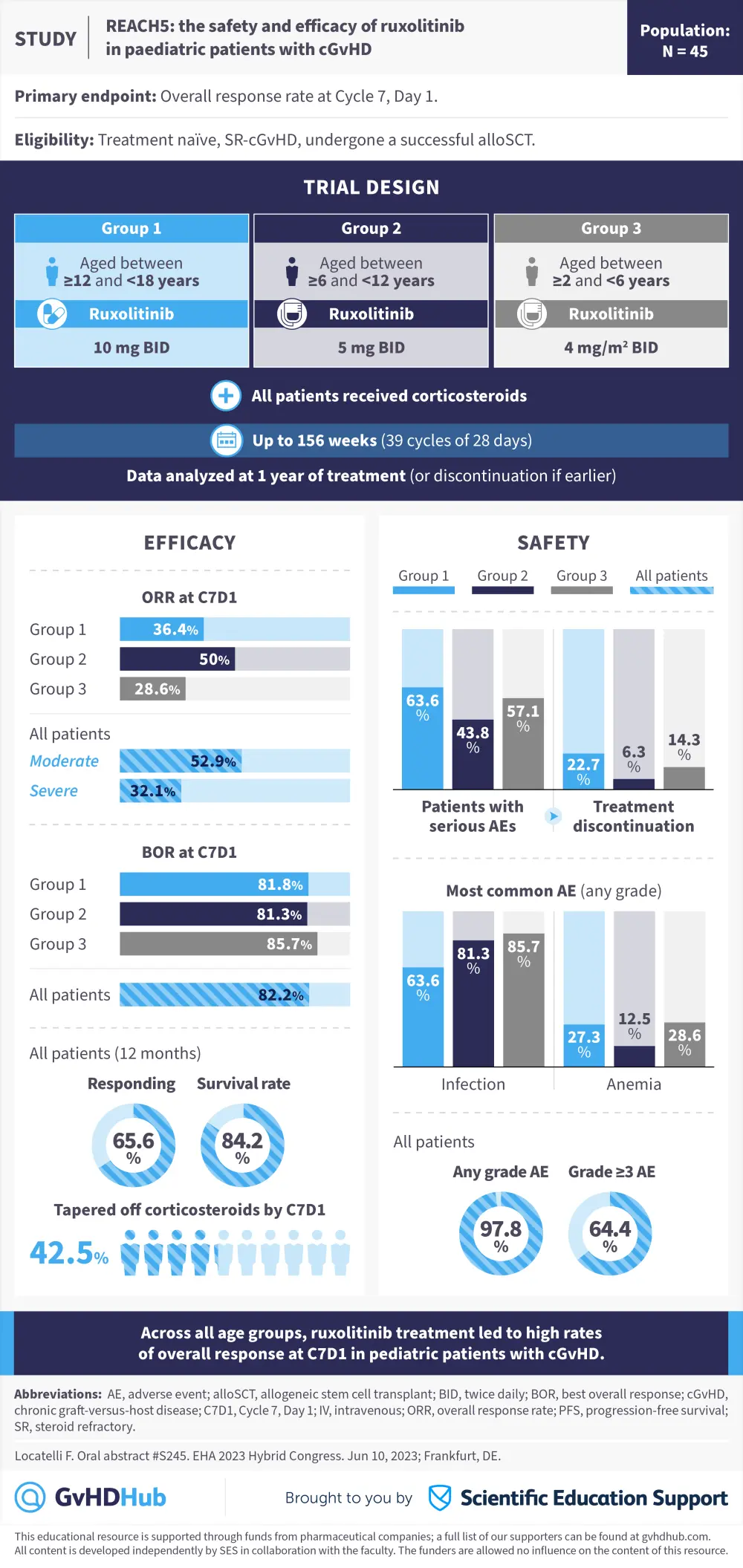
Visual abstract | REACH5: Ruxolitinib in pediatric cGvHD
The GvHD Hub is pleased to present a visual abstract representing preliminary data from the phase II REACH5 trial (NCT03774082) evaluating ruxolitinib, a Janus kinase inhibitor, for use in pediatric patients with chronic graft-versus-host disease (cGvHD).1
Occurrence of cGvHD in pediatric patients can impact quality of life and morbidity.1 In these patients, systemic corticosteroids are the current standard-of-care treatment and there are limited options for second-line therapies.1 In REACH5, pediatric patients with treatment-naïve or steroid-refractory cGVHD were treated with ruxolitinib plus corticosteroids. Patients were split into three groups according to age:
- Group 1: ≥12yrs – <18yrs
- Group 2: ≥6yrs – <12yrs
- Group 3: ≥2yrs – <6yrs
The majority of patients in all groups were male. At the data cutoff, 27.3%, 31.3%, and 57.1% of patients in Groups 1, 2, and 3 were ongoing in the treatment phase, respectively. While on treatment or within 30 days of the last dose, 27.3%, 18.8%, and 14.3% of patients in Groups 1, 2, and 3 had died, respectively.1
The safety profile of ruxolitinib in this study was similar to previous studies in pediatric patients, with ruxolitinib treatment leading to high overall response rates across all age groups.1
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content


