All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional.
The gvhd Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the gvhd Hub cannot guarantee the accuracy of translated content. The gvhd and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The GvHD Hub is an independent medical education platform, sponsored by Medac and supported through grants from Sanofi and Therakos. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View GvHD content recommended for you
Positive results from the phase III REACH3 trial of ruxolitinib for patients with SR cGvHD
Systemic steroids are a standard first-line therapy for chronic graft-versus-host disease (cGvHD), however, up to 50% of patients will eventually become steroid refractory (SR) or steroid dependent. Currently there is no standard second-line treatment for these patients.
Ruxolitinib—a first-in-class oral inhibitor of the tyrosine kinases JAK1 and JAK2—is approved for the treatment of polycythemia vera and myelofibrosis in adults and acute GvHD in both adults and pediatric patients ≥ 12 years of age.1 The REACH3 trial investigated ruxolitinib against the best available therapies (BAT) for patients with SR cGvHD and was reported on by Robert Zeiser at the 62nd American Society of Hematology (ASH) Annual Meeting and Exposition.2 This article will provide a summary of the data presented.
Update: In July 2021, the results of this trial were published in the New England Journal of Medicine with an additional subgroup analysis that showed higher overall response rates for patients treated with ruxolitinib regardless of the individual organs involved at baseline.3
Trial design
The REACH3 trial (NCT03112603) is a phase III, randomized trial of 329 patients with SR cGvHD.
Patients were randomized to receive either ruxolitinib or their physician’s choice of BAT, as shown in Figure 1.
Physician’s choice of BAT could include extracorporeal photopheresis, low-dose methotrexate, mycophenolate mofetil, mTOR inhibitors (everolimus or sirolimus), infliximab, rituximab, pentostatin, imatinib, or ibrutinib.
Eligibility criteria for inclusion in the trial:
- Age ≥ 12 years
- Received allogeneic hematopoietic cell transplant
- Moderate or severe SR/steroid-dependent cGvHD, defined as:
- Lack of response or disease progression after prednisone ≥ 1 mg/kg/day for ≥ 1 week, or
- Disease persistence without improvement with prednisone > 0.5 mg/kg/day or 1 mg/kg/every other day for ≥ 4 weeks or
- Increase in prednisone dose to > 0.25 mg/kg/day after two unsuccessful attempts to taper the dose2
- Myeloid and platelet engraftment
Figure 1. REACH3 trial design2
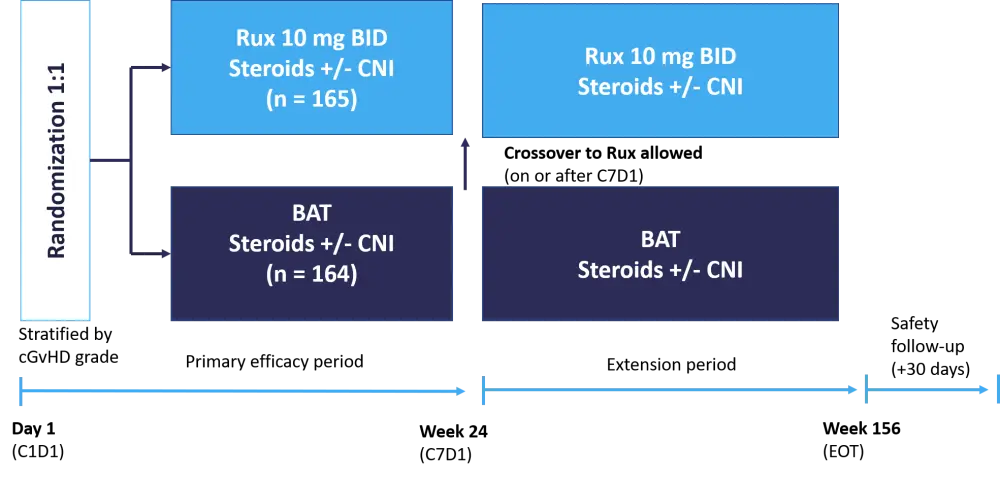
The primary endpoint was overall response rate (ORR) at Week 24.
Secondary endpoints included:
- Failure free survival (FFS)
- Modified Lee Symptom Scale (mLSS) at Week 24
- Best overall response
- Duration of response
The baseline patient characteristics were well balanced between arms (Table 1). The median time from onset of cGVHD to randomization was 24.9 weeks (range, 1.0–288.1) in the ruxolitinib arm and 21.4 weeks (range, 1.4–278.1) in the BAT arm. The two treatment arms were well balanced, and the majority of patients were between 18 and 65 years old and with severe cGvHD.
Table 1. Baseline patient characteristics2
|
BAT, best available therapy; cGvHD, chronic graft-versus-host disease; CMV, cytomegalovirus; mLSS, modified Lee Symptom Score. *Protocol deviation as the inclusion criterium was for moderate to severe GvHD. |
||
|
Characteristic |
Ruxolitinib (n = 165) |
BAT (n = 164) |
|---|---|---|
|
Median age, years (range) |
49.0 (13.0−73.0) |
50.0 (12.0–76.0) |
|
12 to < 18 years, % |
2.4 |
4.9 |
|
18 to 65 years, % |
86.7 |
81.7 |
|
> 65 years, % |
10.9 |
13.4 |
|
cGvHD severity, % |
|
|
|
Mild* |
0 |
0.6 |
|
Moderate |
41.2 |
44.5 |
|
Severe |
58.8 |
54.9 |
|
Total mLSS score, median (range) |
18.67 (0–79.6)† |
18.54 (0.7–54.4)‡ |
|
Median time from cGvHD onset to randomization, weeks (range) |
24.9 (1.0–288.1) |
21.4 (1.4–278.1) |
|
Stem cell source, % |
|
|
|
Peripheral blood |
85.5 |
79.9 |
|
Bone marrow |
13.3 |
18.9 |
|
Single cord blood |
1.2 |
1.2 |
|
Donor type, %§ |
|
|
|
Related |
54.5 |
52.1 |
|
Unrelated |
45.5 |
47.9 |
|
Donor/recipient CMV status, % |
|
|
|
–/– |
30.9 |
27.4 |
|
–/+ |
18.2 |
17.1 |
|
+/– |
9.7 |
10.4 |
|
+/+ |
40.6 |
44.5 |
|
Unknown| |
0.6 |
0.6 |
Patient disposition
Treatment was discontinued in 49.7% of patients treated with ruxolitinib and 74.4% of patients treated with BAT.
Main reasons for discontinuation were lack of efficacy (ruxolitinib, 14.5% compared with BAT, 42.7%) and adverse events (ruxolitinib, 17% compared with BAT, 4.9%). In total, 37.2% of patients on BAT crossed over to the ruxolitinib treatment arm.
Key findings
- ORR (complete response [CR] and partial response [PR]) at 24 weeks: 49.7% with ruxolitinib compared with 25.6% for BAT (odds ratio, 2.99; 95% CI, 1.86−4.80; p < 0.0001)
- Ruxolitinib: CR, 6.7% and PR, 43.0%
- BAT: CR, 3.0% and PR, 22.6%
- FFS was not reached for ruxolitinib vs 5.7 months for BAT (hazard ratio, 0.370; 95% CI 0.268−0.510; p < 0.0001)
- mLSS at 24 weeks: 24.2% in the ruxolitinib group compared with 11.0% for BAT (odds ratio, 2.62; 95% CI, 1.42−4.82; p = 0.0011)
- Best overall response (patients achieving CR or PR at any time up to Week 24): ruxolitinib group achieved 76.4% compared with 60.4% in the BAT group
- Ruxolitinib: CR, 12.1% and PR, 64.2%
- BAT: CR, 6.7% and PR, 53.7%
- Median duration of response was not reached in the ruxolitinib arm and 6.24 months in the BAT arm
The safety profile for the two groups is shown in Figure 2. Median duration of exposure was 41.3 weeks (range, 0.7–127.3) in the ruxolitinib group and 24.1 weeks (range, 0.6–108.4) for BAT. The number of deaths between the two treatment arms was not significantly different (ruxolitinib, 31 vs BAT 27; the most common causes of death were cGvHD and infections).
Figure 2. Safety profile of the two treatment arms up to 24 weeks
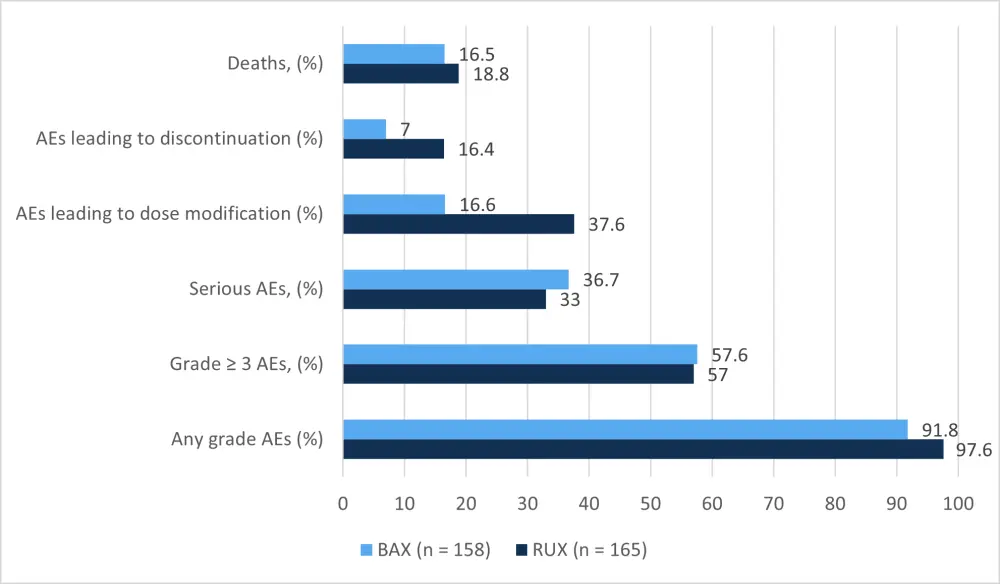
The safety profile of ruxolitinib was consistent with previous reports, with anemia and thrombocytopenia being the most frequent adverse events (AEs). While the majority of AEs were similar in incidence between the two treatment arms, anemia and thrombocytopenia were more common in the ruxolitinib group (Table 2).
Table 2. Adverse events (≥ 10%) up to Week 24 in the two treatment arms2
|
ALT, alanine aminotransferase; BAT, best available therapy; Rux, ruxolitinib. |
||||
|
Event, % |
Rux (n = 165) |
BAT (n = 158) |
||
|---|---|---|---|---|
|
|
Any grade |
Grade ≥ 3 |
Any grade |
Grade ≥ 3 |
|
Hematologic |
|
|
|
|
|
Anemia |
29.1 |
12.7 |
12.7 |
7.6 |
|
Thrombocytopenia |
21.2 |
15.2 |
14.6 |
10.1 |
|
Neutropenia |
10.9 |
8.5 |
5.1 |
3.8 |
|
Gastrointestinal |
|
|
|
|
|
Diarrhea |
10.3 |
0.6 |
13.3 |
1.3 |
|
Nausea |
9.1 |
0 |
10.1 |
1.3 |
|
Infections |
|
|
|
|
|
Pneumonia |
10.9 |
8.5 |
12.7 |
9.5 |
|
Lab abnormalities |
|
|
|
|
|
ALT increased |
15.2 |
4.2 |
4.4 |
0 |
|
Creatinine increased |
13.9 |
0 |
4.4 |
0.6 |
|
Hypokalemia |
7.9 |
1.8 |
10.1 |
4.4 |
|
Other |
|
|
|
|
|
Hypertension |
15.8 |
4.8 |
12.7 |
7.0 |
|
Pyrexia |
15.8 |
1.8 |
9.5 |
1.3 |
|
Cough |
10.3 |
0 |
7.0 |
0 |
|
Fatigue |
10.3 |
0.6 |
7.6 |
1.9 |
In terms of infections, there was no significant difference between the treatment arms in the number of viral or bacterial infections observed (ruxolitinib: viral 33.9%, bacterial 27.9%; BAT: viral 29.1%, bacterial 25.9%). A higher percentage of patients in the ruxolitinib group experienced fungal infections (11.5%) compared with the BAT group (5.7%). Cytomegalovirus infection/reactivation occurred in 5.5% of patients in the ruxolitinib arm and 8.2% in the BAT arm.
Conclusion
This is the first successful randomized, phase III study in adolescents and adults with SR cGvHD in which ruxolitinib demonstrated greater efficacy compared with the BAT. The trial met its primary endpoint, with a significantly higher ORR observed with ruxolitinib compared with BAT. Treatment with ruxolitinib (compared with BAT) was also associated with a significant improvement in patient-reported symptoms, FFS, and a higher rate of best overall response. The safety profile of ruxolitinib was deemed acceptable and consistent with the known safety profile. These trial results will form the basis of a submission to the U.S. Food and Drug Administration (FDA) for the approval of ruxolitinib in patients with steroid-refractory or steroid-dependent cGvHD.
Expert Opinion
 Robert Zeiser
Robert ZeiserReferences
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

