All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional.
The gvhd Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the gvhd Hub cannot guarantee the accuracy of translated content. The gvhd and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The GvHD Hub is an independent medical education platform, sponsored by Medac and supported through grants from Sanofi and Therakos. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View GvHD content recommended for you
Updated guidelines for post-HSCT vaccination
Introduction
At the 5th Annual Meeting of the International Academy for Clinical Hematology (IACH), Ljungman presented the updated vaccine guidelines for posttransplant patients.1
Since the guidelines were last revised in 2017 and published in 2019, new vaccines have been developed including the Covid-19 vaccine, recombinant zoster vaccine, and pneumococcal vaccines. In patients who are undergoing or have undergone a hematopoietic stem cell transplant (HSCT), there are considerations before vaccination, such as the transplant type and conditioning regime used.1
The decision to vaccinate1
Before vaccinating a patient who has undergone HSCT, the immune status of the patient and risk of severe disease versus the possible benefit of an adequate response should be considered. Factors that can affect immune status are shown in Figure 1.
Figure 1. Factors influencing HSCT patient immune status*
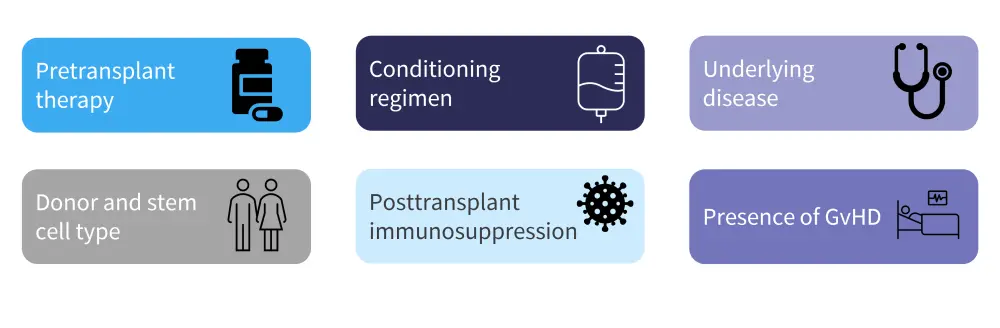
GvHD, graft-versus-host disease; HSCT, hematopoietic stem cell transplant.
*Adapted from Ljungman.1
Another important consideration, specifically in allogeneic transplant, is the transfer of donor immune cells donor to the recipient; pre-donation vaccination may improve transfer of immune cells in this case. However, as allogeneic transplant can result in the removal of immunological memory from the recipient and a booster dose does provide adequate protection, full primary schedules of vaccination are required soon after transplant.1
Both inactivated and live vaccines can pose risks to transplant recipients:
- With inactivated vaccines, there is no evidence of major risks from direct side effects, although local and systemic side effects can occur, and they are low risk for immune activation complications.
- With live vaccines, there is a risk of vaccine-induced disease, particularly in patients with suppressed T-cell immunity, and immune activation complications including fatal incidences, have been documented, although the risk is still low.
Vaccination schedules1
Vaccination schedules using fixed time points are more easily administered and controlled, with all patients being managed the same; there is also a reduced risk of patients missing vaccinations. Using flexible time points based on immunological response (total CD4+, CD3+, and circulating B cells) may result in missed vaccinations and patients becoming lost to follow-up; however, there is a higher likelihood of response with a schedule that is adapted to the patient and potentially less side effects.
Although many vaccines have robust toxicity data, there is a lack of efficacy data available for posttransplant patients. Efficacy data is based on surrogate endpoints such as immune response rather than protection offered to the patient.
Vaccine recommendations1
Table 1 highlights vaccination recommendations based on the previous 2019 guidelines.1
Table 1. Vaccine recommendations for posttransplant patients*
|
cGvHD, chronic graft-versus-host-disease; Hib, haemophilus influenzae type B; MMR, measles, mumps, and rubella. |
|||
|
Vaccine |
Type |
Recommended |
Schedule |
|---|---|---|---|
|
Tetanus toxoid + diphtheria toxoid |
Inactivated |
Yes |
Three doses, starting 6 months after |
|
Influenza |
Inactivated |
Yes |
4–6 months after transplant (seasonal) |
|
Poliovirus |
Inactivated |
Yes |
Three doses, starting 6–12 months |
|
Conjugated Hib |
Inactivated |
Yes |
Three doses, starting 6–12 months after transplant |
|
Pneumococcal conjugate |
Inactivated |
Yes |
Three doses, starting 3–6 months after |
|
Pneumococcal polysaccharide |
Inactivated |
Yes |
Booster at 12 months in patients without GvHD |
|
Acellular pertussis |
Inactivated |
Yes |
Started 6–12 months after transplant in children <7 years |
|
Hepatitis B |
Inactivated |
Yes |
Started 6–12 months after transplant† |
|
Papillomavirus |
Inactivated |
Yes |
Three doses, starting 6–12 months |
|
Meningococcal conjugate |
Inactivated |
Yes |
Two doses, starting 6 months after |
|
COVID-19 |
Inactivated |
Yes |
Limited data currently |
|
MMR |
Live |
Individual consideration |
For children and seronegative adults, at |
|
Varicella |
Live |
Individual consideration |
For seronegative adults, at least 24 |
|
Zoster |
Live |
No |
|
Updated recommendations
Influenza vaccines
In the previous guidelines, influenza vaccination was included for its possible benefit to HSCT patients. There are now data to support this, although there is no obvious benefit to using adjuvanted vaccines versus non-adjuvanted. Response rates vary from 10% to 74% for one dose of the adjuvanted trivalent vaccine and from 44% to 64% for the H1N1 vaccine (when given more than 6 months after transplant), with response improving as time after transplant increases.1 Data on the benefit of giving a second dose is unclear. Studies have shown that vaccination reduces the risk of influenza in patients who have undergone HSCT as well as the risk for progression to pneumonia; it was also shown to reduce viral load and the risk of intensive care unit admission.
Varicella-zoster vaccines
In posttransplant patients, use of the live varicella vaccine can cause fatal infections; however, the recombinant zoster vaccine has now been approved in Europe for use in these patients. In a phase III study in patients with hematologic malignancies, 40 of whom had previously received a recombinant allogeneic HSCT, vaccine efficacy was 87.2% in the post-hoc analysis of the whole study population. Additionally, in a prospective observational study in 158 patients, some patients experienced breakthroughs after discontinuation of antiviral prophylaxis, which was fatal in one patient. This suggest further clinical trials of varicella vaccination in this patient group are warranted.1
Covid-19
Covid-19 vaccination can potentially increase the risk of eliciting or worsening graft-versus-host-disease.1 Data showed that use of a third dose increased antibody levels and T-cell responses after vaccination. A three dose schedule is now recommended posttransplant for patients who have already been vaccinated pretransplant; however several countries have now changed their recommendations to five doses for posttransplant patients. Further clinical trials are needed to determine safety concerns for these patients and whether it is beneficial to use the same vaccine type for every dose. The use of Covid-19 vaccination in HSCT patients has been previously reported by the GvHD Hub.1
Pneumococcal vaccines
Two new pneumococcal vaccines have been licensed in Europe, a 20-valent vaccine and a 15-valent vaccine. Both showed similar immunogenicity to the pneumococcal conjugate vaccine; however, the new vaccines are not included in the guidelines. In a study of 100 patients, 66% received the recommended dose schedule for pneumococcal vaccines; retainment of anti-pneumococcal antibodies in these patients was partial and additional information is needed on optimum dose numbers.1
Conclusion
According to international recommendations, all HSCT recipients should receive vaccinations based on their individual needs; the guidelines will continue to be updated as new vaccines are developed. Ljungman suggested that additional vaccine doses should be considered to enable long-term protection. Further clinical trials for all vaccine types in posttransplant patients would be beneficial to provide efficacy data, particularly in patients with GvHD, enabling healthcare professionals to make informed decisions when treating patients undergoing HSCT.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content



