All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional.
The gvhd Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the gvhd Hub cannot guarantee the accuracy of translated content. The gvhd and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The GvHD Hub is an independent medical education platform, sponsored by Medac and supported through grants from Sanofi and Therakos. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View GvHD content recommended for you
Case study | 51-year-old with chronic GvHD with no improvement after a week on prednisone
Featured:
First-line therapy for patients with graft-versus-host disease (GvHD) is treatment with steroids; however, ~50% of patients do not respond to initial treatment.1 To explore how to approach these patients, we spoke to Arnon Nagler, who has provided his opinion on how to treat a particular patient with chronic GvHD who was not responding to prednisone.
Case study
The initial characteristics of the patient in question are presented in Figure 1.
Figure 1. Patient characteristics and treatment history
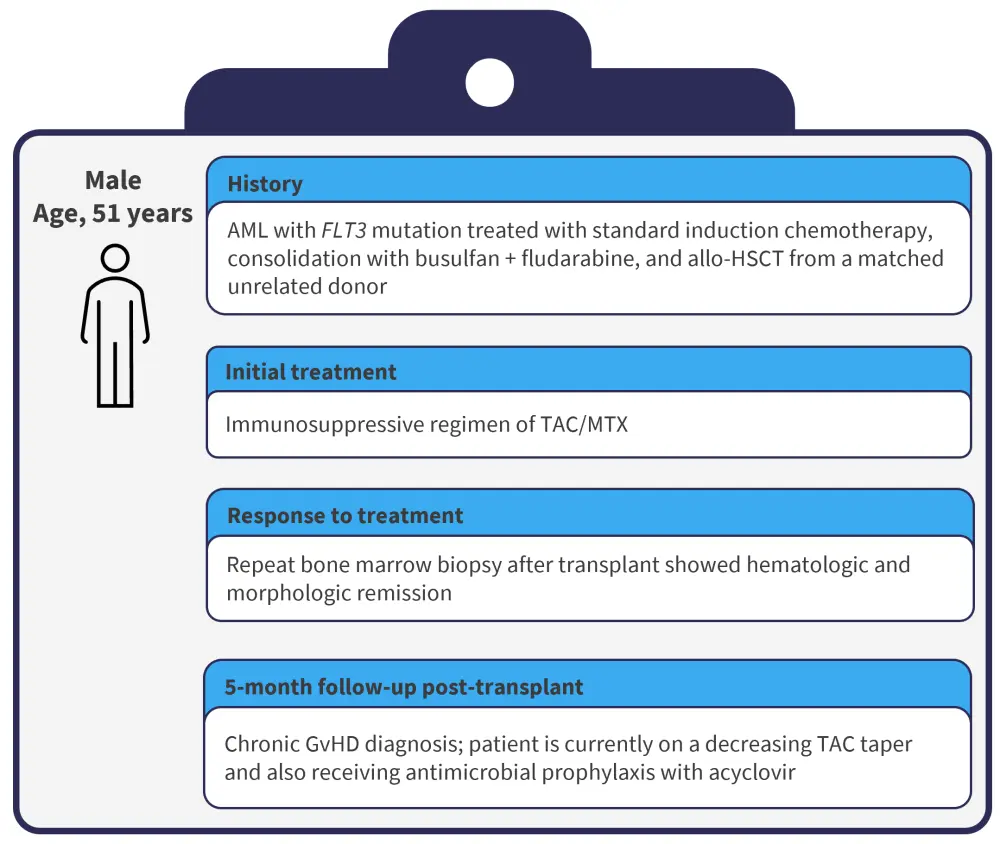
Allo-HSCT, allogeneic hematopoietic stem cell transplant; AML, acute myeloid leukemia; GvHD, graft-versus host disease; MTX, methotrexate; TAC, tacrolimus.
5-month follow-up post-transplant
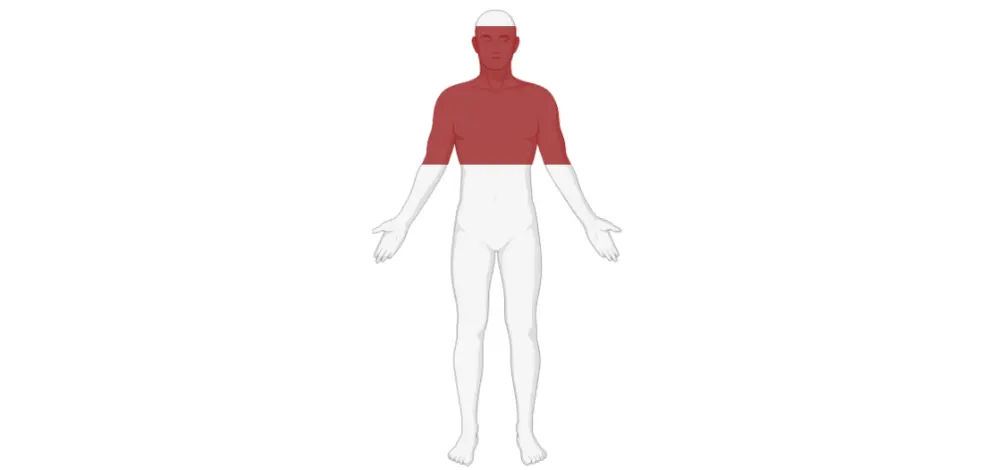
- On examination, the patient had a maculopapular rash across both upper limbs and chest and oral lesions suggestive of lichen planus (Figure 2).
- The patient reported symptoms began 3−4 months post allogeneic hematopoietic stem cell transplant (allo-HSCT) and was unsuccessful in managing with over-the-counter solutions.
- During the visit, the patient noticeably rubbed their eyes a lot. He said his eyes have been feeling dry but not so much as to affect his daily activities.
- According to the National Institute of Health global severity score of chronic GvHD,2 a diagnosis of moderate chronic GvHD was confirmed (based on skin score, 2; mouth score, 1)
Figure 2. Affected area of the maculopapular rash and oral lesions
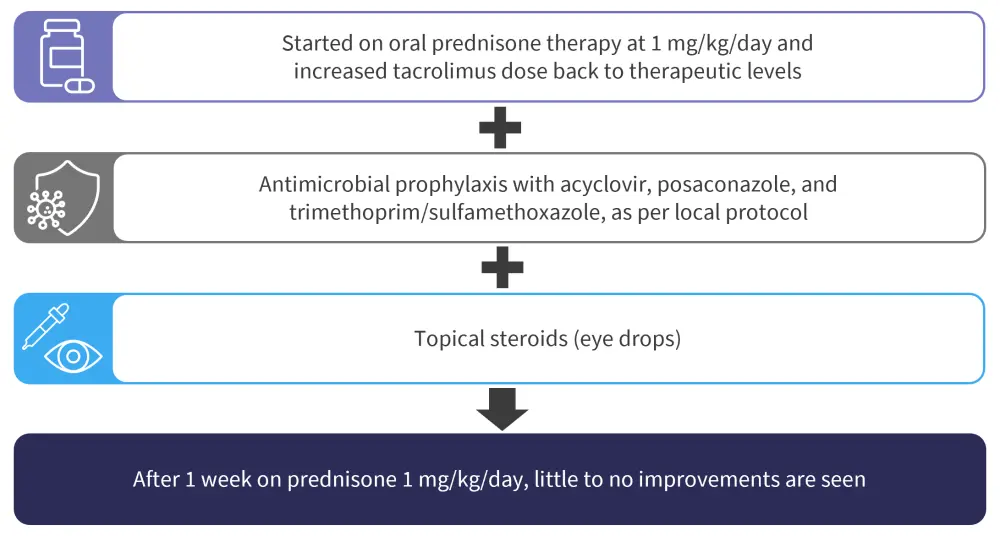
Initial treatment for chronic GvHD
Figure 3. Administered therapy and outcome
Question for expert

Expert Opinion
In summary
The patient is young and has developed moderate chronic GvHD with skin mucosal and ophthalmic involvement early post-allo-HSCT (5 months) during tapering of post-transplant immunosuppression. The patient has now failed to respond to 1 mg/kg steroid treatment alongside increased tacrolimus dosing (back to therapeutic levels).
Patient next steps
Figure 4. Treatment plan
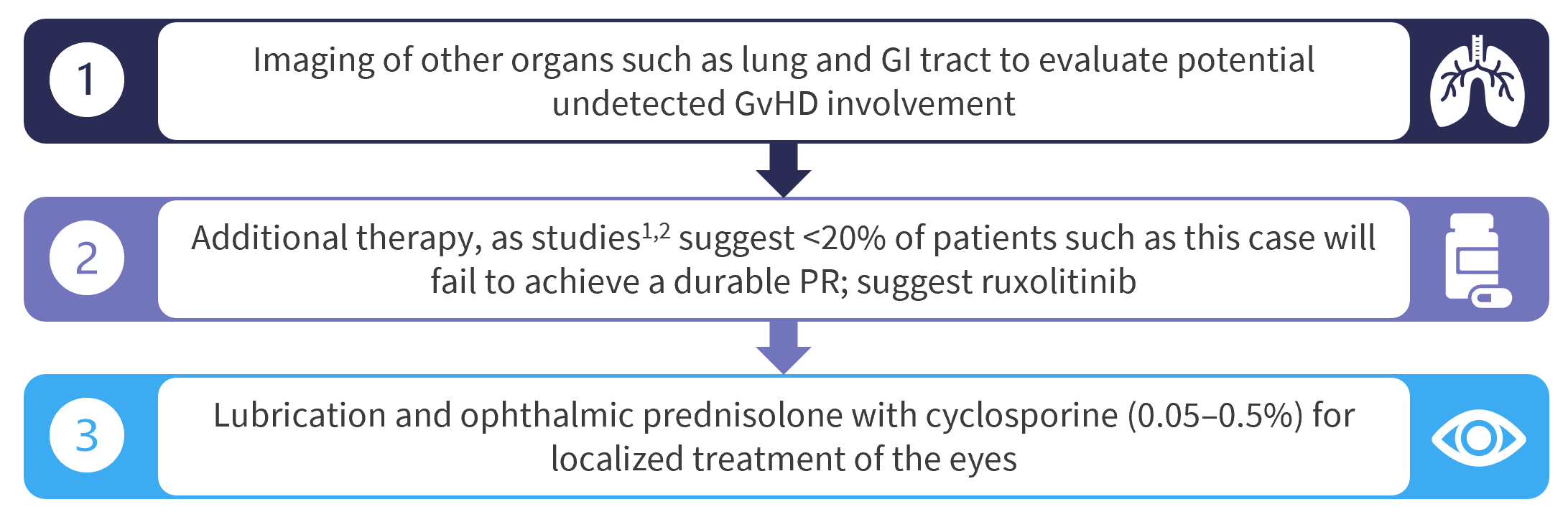
GI, gastrointestinal; PR, partial response.
 Arnon Nagler
Arnon NaglerReferences
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
Which consideration most strongly guides your decision to escalate therapy in SR-aGvHD?

