All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional.
The gvhd Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the gvhd Hub cannot guarantee the accuracy of translated content. The gvhd and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The GvHD Hub is an independent medical education platform, sponsored by Medac and supported through grants from Sanofi and Therakos. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View GvHD content recommended for you
Ruxolitinib treatment pre-, during-, and post-transplant for patients with MF
Myelofibrosis (MF) is clonal myeloproliferative disorder characterized by splenomegaly, bone marrow fibrosis, and risk of transformation to acute myeloid leukemia. Patients with intermediate- and high-risk disease benefit more from hematopoietic stem cell transplant (HSCT), while patients with asymptomatic favorable-risk disease are observed and those with symptomatic favorable-risk may be given a JAK 1/2 inhibitor, such as ruxolitinib.
Retrospective studies of ruxolitinib prior to transplantation for intermediate/high risk MF suggest that patients who respond in terms of splenomegaly have improved transplant outcomes.1 Additionally, ruxolitinib has been approved by the U.S. Food and Drug Administration (FDA) for posttransplant treatment of graft-versus-host disease (GvHD) in chronic GvHD (cGvHD) and steroid-refractory acute GvHD (aGvHD). A previous phase I study showed peritransplant use of ruxolitinib was safe and well tolerated, producing promising survival rates in patients with MF.2 There is variation in practice regarding tapering ruxolitinib prior to HSCT, and a question around the safety and efficacy of ruxolitinib throughout the transplant setting.1
At the 63rd American Society of Hematology (ASH) Annual Meeting and Exposition, Gabriela Hobbs presented interim data from a phase II study investigating the safety and efficacy of ruxolitinib treatment pre-, during-, and post-HSCT in patients with primary and secondary MF (NCT03427866). We summarize key findings below.1
Study design
This was a phase II, multi-center, investigator-initiated trial, with ruxolitinib treatment given up to 1-year following HSCT. A summary of the study design is illustrated in figure 1.
Figure 1. Study design*
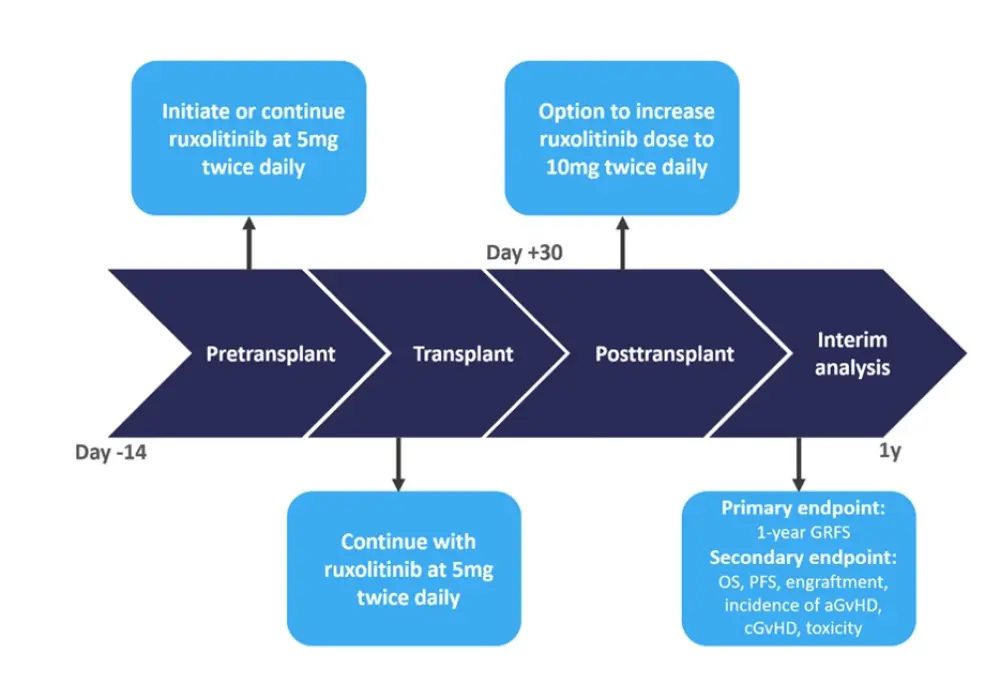
aGvHD, acute graft versus host disease; cGvHD, chronic graft versus host disease; GRFS, GvHD free and relapse free survival; OS, overall survival, PFS, progression-free survival.
Conditioning consisted of a reduced intensity regimen with fludarabine (30mg/m2/day × 5 days) and melphalan (100mg/m2 or 140mg/m2 × 1 day). GvHD prophylaxis consisted of methotrexate and tacrolimus.
*Adapted from Hobbs1
Inclusion criteria for this study included:
- Age ≥18 years
- World Health Organisation (WHO) defined primary or secondary myelofibrosis
- DIPSS+ intermediate-1 (with adverse risk molecular markers) or > int-2
Results
Out of a total of 26 patients enrolled, the majority were male (75%), had DIPSS+ intermediate-2 or high-risk (92%) classification, and half of patients were receiving ruxolitinib before transplant (Table 1).
Table 1. Baseline characteristics (N = 26)*
|
DIPSS, dynamic international prognostic scoring system for myelofibrosis; HLA, human leukocyte antigen; HSCT, hematopoietic stem cell transplant. |
|
|
Characteristic, % (unless otherwise stated) |
N = 26 |
|---|---|
|
Median age (range), years |
66 (46–75) |
|
Female |
25 |
|
DIPSS risk |
|
|
Intermediate-1 |
8 |
|
Intermediate-2 |
46 |
|
High-risk |
46 |
|
Receiving ruxolitinib at enrolment |
54 |
|
HLA match |
|
|
8/8 matched related |
77 |
|
8/8 matched unrelated |
19 |
|
<8/8 matched unrelated |
4 |
|
Presence of splenomegaly |
85 |
|
Cytogenetics |
|
|
Normal |
73.1 |
|
Abnormal |
15.4 |
|
Unavailable |
11.5 |
|
Mutational status at HSCT |
|
|
JAK2 |
58 |
|
CALR |
12 |
|
MPL |
12 |
|
ASXL1 |
35 |
As expected, the most common mutation present prior to transplant was JAK2 (58%), while 12% of patients carried CALR mutations and 12% carried MPL, and finally 35% of patients carried ASXL1 mutation.
Safety
No unexpected adverse events were reported, and treatment was well tolerated. The most common Grade 3/4 hematologic events were anemia and thrombocytopenia (table 2).
Table 2. Grade 3/4 hematologic adverse events*
|
AE, adverse event. |
|
|
Grade 3/4 AE, n |
N = 26 |
|---|---|
|
Anemia |
4 |
|
Leukopenia |
2 |
|
Neutropenia |
1 |
|
Hypertriglyceridemia |
1 |
|
Infections |
1 |
|
Kidney infection |
1 |
|
Thrombocytopenia |
3 |
|
Urinary tract infection |
1 |
Engraftment
Hobbs reported engraftment of [defined as absolute neutrophil count >0.5 × 109] in all but one patient (23/24) by Day 30, who then went on to achieve engraftment by Day 60. The median time to neutrophil engraftment was 15 days.
Delayed engraftment of platelets was observed, with 14/23 patients achieving engraftment in the first 30 days (defined as 20 × 109 for the first 13 and >10 × 109 for the rest), while a further four patients achieved engraftment by Day 60, and by Day 150, all patients had achieved engraftment.
Survival outcomes
Clinical outcomes posttransplant with ruxolitinib are summarized in Table 3.
In terms of the primary endpoint, 65% of patients were GvHD free and relapse free at 12 months.
Table 3. Clinical outcomes with post-transplant ruxolitinib treatment*
|
95% CI, 95% confidence interval; aGvHD, acute graft versus host disease; cGvHD, chronic graft versus host disease; GRFS, GvHD free and relapse free survival; NRM; non-relapse mortality; OS, overall survival, PFS, progression-free survival. |
|
|
Outcome (95% CI), % |
N = 26 |
|---|---|
|
1-year GRFS |
65 (39–82) |
|
1-year OS |
77 (52–90) |
|
1-year PFS |
71 (45–86) |
|
1-year cumulative incidence of NRM |
13 (3–30) |
|
1-year cumulative incidence of relapse |
17 (4–38) |
|
6-month grade 2–4 aGvHD incidence |
35 (17–53) |
|
6-month grade 3–4 aGvHD incidence |
4 (0.3–17) |
|
1-year cGvHD incidence |
14 (3–32) |
|
1-year moderate/severe cGvHD incidence |
5 (0.3–22) |
In terms of tolerability, the median number of cycles of ruxolitinib treatment was nine (range, 2–17 cycles), each lasting a month.
Comparison of mutational profile before and after HSCT
Next-generation sequencing was performed before and 100 days post-HSCT. All patients cleared their mutations except for one patient who had detectable JAK2 mutation. However, this patient remained in remission after 1 year.
Conclusion
The interim data from this study provides evidence for the safety of ruxolitinib treatment pre- during and post-HSCT for patients with MF. Notably, PFS, OS, and, GRFS with ruxolitinib treatment were superior to historical observations with minimal incidence of severe aGvHD and cGvHD.
Hobbs concluded by highlighting next steps which include completing this phase II study, which will include 45 patients, followed by further research into in depth testing of clonal dynamics pre and posttransplant to better understand changes to the mutational profile, and be able to predict relapse to enable timely intervention.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
Which consideration most strongly guides your decision to escalate therapy in SR-aGvHD?

