All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional.
The gvhd Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the gvhd Hub cannot guarantee the accuracy of translated content. The gvhd and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The GvHD Hub is an independent medical education platform, sponsored by Medac and supported through grants from Sanofi and Therakos. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View GvHD content recommended for you
Ruxolitinib in combination with low-dose steroids for intermediate- and high-risk aGvHD
The most common first-line treatment for acute graft-versus-host disease (aGvHD) is corticosteroids; however, approximately 50% of patients are refractory to first-line corticosteroid treatment and therefore have poor outcomes.1 At the 64th American Society of Hematology (ASH) Annual Meeting and Exposition, Liu presented results from a multicenter phase II trial (NCT04061876) investigating the use of ruxolitinib in combination with corticosteroids as a novel first-line treatment for aGvHD.1
Study design1
Patients with intermediate- or high-risk aGvHD, as determined by biomarker analysis (REG3a and ST2 were measured at Day 0 and following 4 weeks of treatment), were enrolled in the study (n = 198; Figure 1). Patients in the ruxolitinib group received a reduced dose of steroids every 5 days to achieve a dose of 0.1 mg/kg by Day 30, before being tapered off after 6 weeks. Ruxolitinib was given every day for 90 days and then tapered off.
Figure 1. Study design*
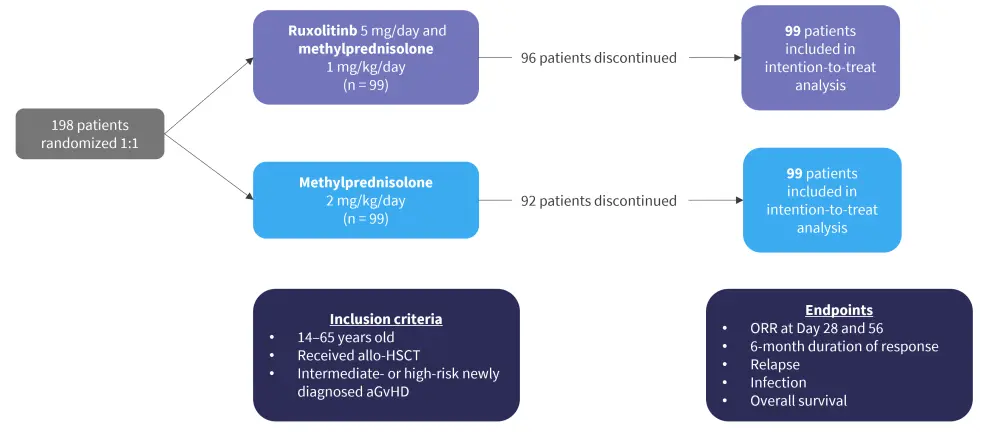
aGvHD, acute graft-versus-host disease; allo-HSCT, allogeneic hematopoietic stem cell transplant; ORR, overall response rate.
*Adapted from Liu.1
Results1
Baseline patient characteristics are shown in Table 1. The majority of patients were male and the most common diagnosis was acute myeloid leukemia.
Table 1. Baseline patient characteristics*
|
ALL, acute lymphoblastic leukemia; AML, acute myeloid leukemia; Bu, busulfan; Cy, cyclophosphamide; CML, chronic myeloid leukemia; Flu, fludarabine; MDS, myelodysplastic syndrome; NHL, non-Hodgkin lymphoma; TBI, total-body irradiation. |
|||
|
Characteristic, % (unless otherwise |
Ruxolitinib/steroids |
Steroids |
p value |
|---|---|---|---|
|
Median age (range), years |
37.0 (14.0–65.0) |
35.0 (14.0–64.0) |
0.324 |
|
Median donor age (range), years |
39.0 (14.0–60.0) |
34.0 (8.0–54.0) |
0.018 |
|
Male |
66.7 |
76.7 |
0.537 |
|
Diagnosis of malignancy |
|
|
0.111 |
|
AML |
54.5 |
40.4 |
|
|
ALL |
28.3 |
34.3 |
|
|
MDS |
5.1 |
11.1 |
|
|
CML |
1.0 |
2.0 |
|
|
NHL |
5.1 |
3.0 |
|
|
Others |
6.0 |
9.1 |
|
|
Median time from diagnosis to |
217 (31–859) |
411 (20–6,256) |
0.064 |
|
Source of donor |
|
|
0.810 |
|
Matched siblings |
13.1 |
12.1 |
|
|
Related haploidentical |
78.8 |
78.1 |
|
|
Unrelated |
8.1 |
9.1 |
|
|
Conditioning |
|
|
0.721 |
|
Modified Bu/Cy |
89.9 |
88.9 |
|
|
Bu/Flu |
7.1 |
9.1 |
|
|
TBI/Cy |
3.0 |
2.0 |
|
The majority of patients in both treatment groups had Grade 2 GvHD, with skin and upper gastrointestinal tract being most common organs involved. GvHD characteristics at the time of patient diagnosis are shown in Figure 2.
Figure 2. A GvHD grade at diagnosis, B organ involvement at diagnosis, and C biomarker risk at diagnosis*
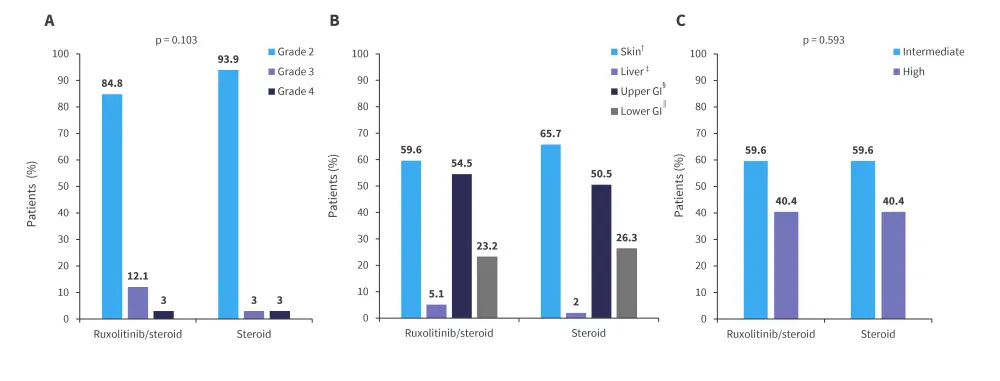
GI, gastrointestinal; GvHD, graft-versus-host disease.
*Adapted from Liu.1
†p = 0.517.
‡p = 0.353.
§p = 0.577.
‖p = 0.678.
Efficacy
The median follow-up time was 601 days. The average time to response was 3 days (range, 1–7 days) and 8 days (range, 1–13 days) in the ruxolitinib and steroid only group, respectively; overall response rates (ORRs) are shown in Figure 3. The rate of failure-free survival was found to be significantly higher in the ruxolitinib group compared with the steroid only group (320 days vs 113 days; hazard ratio [HR], 2.11; 95% confidence interval [CI], 1.35–3.31).
Figure 3. ORR at A Day 28 and B Day 56*
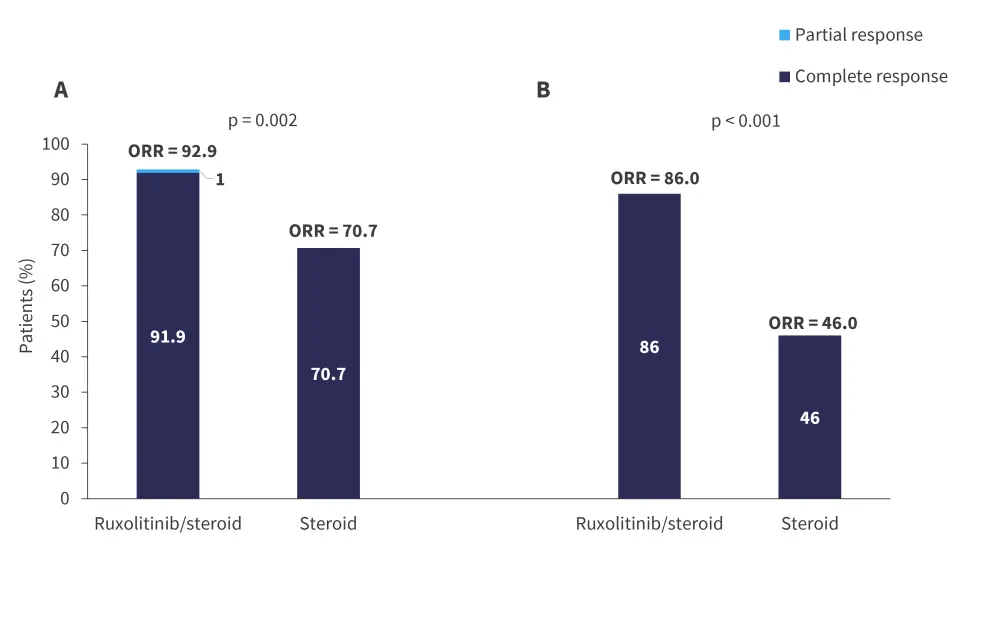
ORR, overall response rate.
*Adapted from Liu.1
At Day 28, older patients (50–65 years) had a significantly higher ORR in the ruxolitinib group compared with the steroid only group (100% vs 60%; p = 0.029). Patients with Grade 3–4 aGvHD also had a higher ORR with ruxolitinib compared with steroids (86.7% vs 33.3%; p = 0.031). The duration of steroid treatment was longer in patients in the steroid only group compared with the ruxolitinib group (48.7 days vs 29.4 days; p = 0.032). Two-year non-relapse mortality was comparable between groups. The Kaplan-Meier 48-month survival proportions were 69.1% and 76.5% for the ruxolitinib and steroid only group, respectively.
Safety
The rate of Grade 3 and 4 severe adverse events was similar between the groups, with 87.9% and 85% of patients experiencing a severe event in the ruxolitinib and steroid only group, respectively; however, Grade 4 events were more common in the steroid only group (50.5% vs 26.3%). Grade 3 and 4 thrombocytopenia occurred in 56.5% and 69.7% of patients treated with ruxolitinib and steroids alone, respectively. Rates of Grade 3 and 4 infections were higher in patients treated with steroids only, there was also no significant difference in the rates of cytomegalovirus and Epstein-Barr virus between treatment groups; the infection rates of both treatment groups are shown in Figure 4.
Figure 4. Rates of infection*
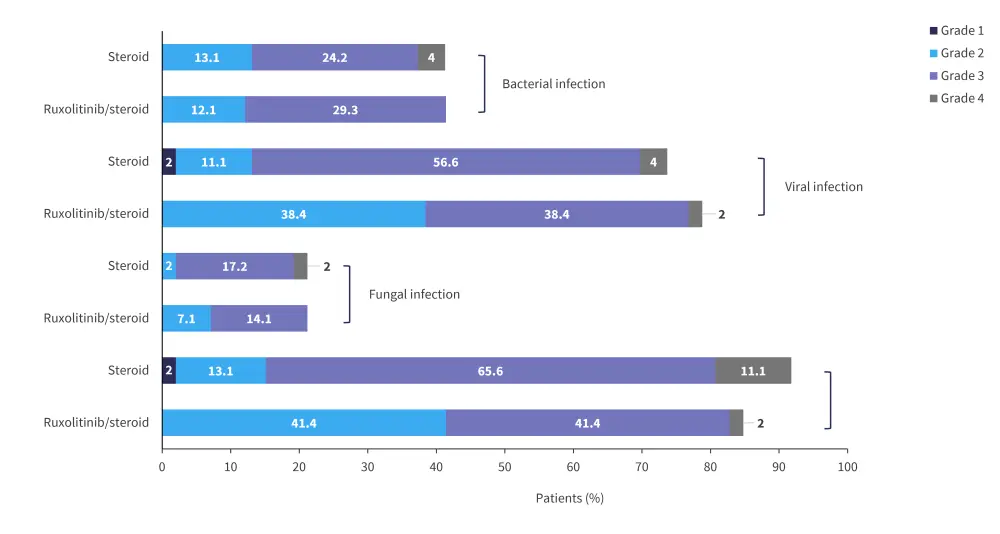
*Adapted from Liu.1
Conclusion1
These data demonstrate that combination low-dose ruxolitinib + steroids can produce high response rates in patients with intermediate- and high-risk aGvHD, with similar safety signals steroid monotherapy. Failure-free survival was significantly higher in the ruxolitinib group compared with the steroids only group; furthermore, reduced cumulative use of steroids was shown to reduce the incidence of side effects and improve patient quality of life. Based on these findings, ruxolitinib with low-dose steroids represents a potential effective treatment option for patients with aGvHD.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
Which consideration most strongly guides your decision to escalate therapy in SR-aGvHD?



