All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional.
The gvhd Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the gvhd Hub cannot guarantee the accuracy of translated content. The gvhd and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The GvHD Hub is an independent medical education platform, sponsored by Medac and supported through grants from Sanofi and Therakos. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View GvHD content recommended for you
ROCKstar: Belumosudil in chronic GvHD, an update from ASH 2021
Update/disclaimer: This article has been modified from that published on May 19, 2021, to include updated data from Cutler and colleagues’ recent publication in the journal Blood1; in addition to a description of the mechanism of action of belumosudil, and an interview from Aleksandr Lazaryan, from the 63rd American Society of Hematology (ASH) Annual Meeting and Exposition.
Graft-versus-host disease (GvHD) is an immune-mediated disorder that can occur after a stem cell transplantation, whereby the T cells of the donor recognize the recipients’ cells as foreign and therefore try to destroy them by releasing cytokines. This results in inflammation, fibrosis, and widespread tissue and organ damage. While the acute version of the disease usually occurs in the first 10–100 days post-transplant, chronic GvHD (cGvHD) normally begins 90–600 days post-transplant and affects up to 70% of all allogeneic stem cell transplant recipients. cGvHD is the leading cause of morbidity, late non-relapse mortality, and impaired quality of life after allogeneic hematopoietic stem cell transplantation.1
The Rho-associated coiled-coil kinase 2 (ROCK2) pathway is accountable for the regulation of type 17 helper T cells (Th17 cells)/regulatory T cells (Treg cells) and is therefore a key driver in the pro-inflammatory and profibrotic pathways that are triggered during cGvHD.1
During the pro-inflammatory immune response (Figure 1A), ROCK2 interacts with phosphorylated STAT3 and leads to the formation of the JAK2/STAT3 complex in Th17 and follicular helper T cells.2 The JAK2/STAT3 complex can interact with transcription factors such as IRF4, RORγT, and Bcl6, which lead to the upregulation of Th17 cells and the production of the pro-inflammatory cytokines; IL-17, and IL-21. These cytokines can cause the activation of B cells and monocytes/macrophages, which results in the excessive production of autoantibodies and the secretion of profibrotic factors, such as lysophosphatidic acid (LPA) and tumor growth factor-β (TGF-β). This pro-inflammatory environment downregulates the number and function of immunosuppressive Treg cells, thereby sustaining chronic inflammation.2
Profibrotic factors can also activate the ROCK2 signaling pathway and its numerous downstream substrates, which results in the polymerization of G-actin to F-actin and the formation of contractile fibers (Figure 1B).2 ROCK2-induced actin polymerization releases myocardin-related transcription factor (MRTF), which upregulates profibrotic genes, such as collagen, α-SMA, CTGF, and TGF-β, resulting in alterations in cellular structure, increased tissue stiffness, and the differentiation of fibroblasts into myofibroblasts.2
Inhibition of ROCK2 is therefore an attractive option for the treatment of cGvHD as it can rebalance the immune responses through the downregulation of pro-inflammatory Th17 cells and increasing Treg cells, as well as reducing the fibrotic process. Inhibition of ROCK2 also allows JAK3 to phosphorylate STAT5, which acts on the transcription factor Foxp3 to upregulate the expression of IL-10 and increase the number of Treg cells (Figure 1C).2
Figure 1. ROCK2 signaling pathway and its inhibition by belumosudil*
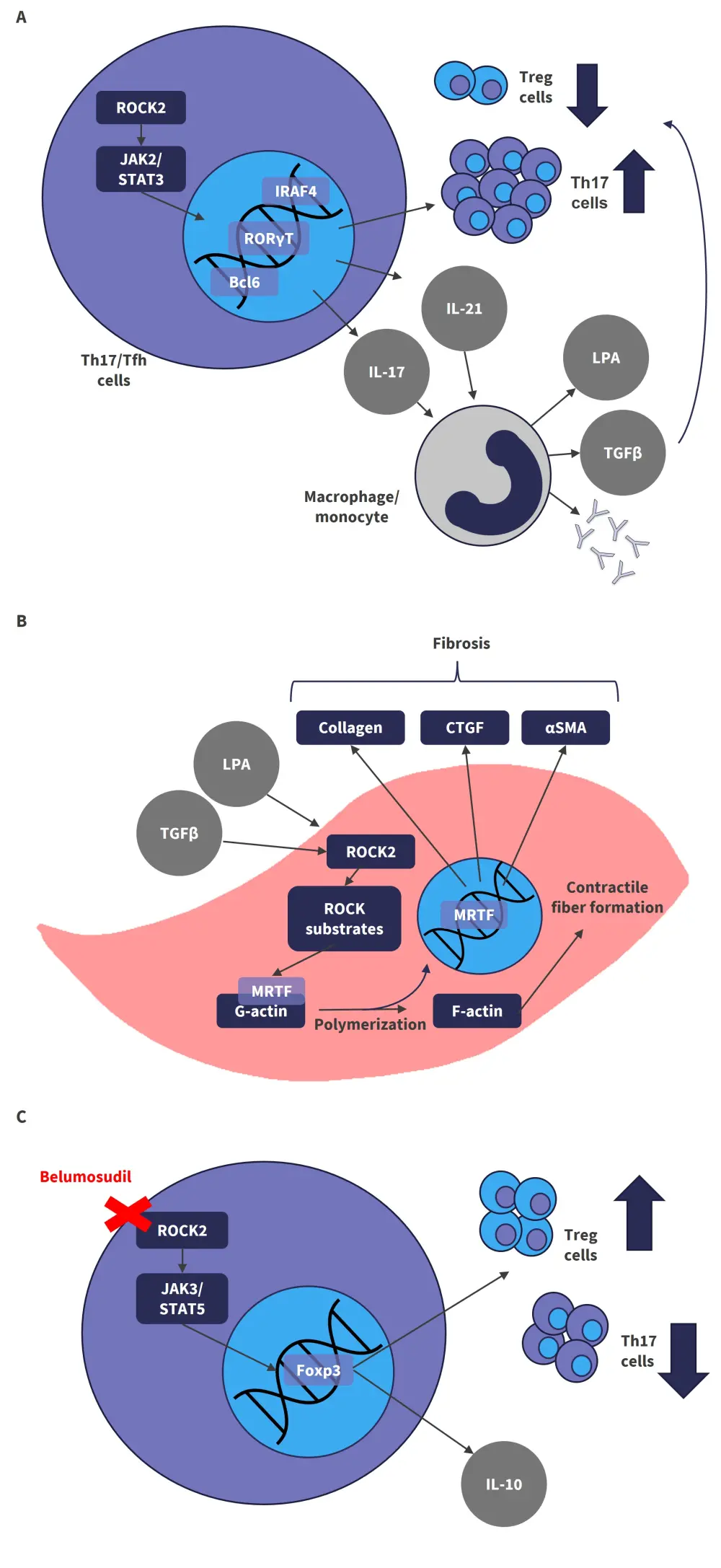
The effects of ROCK2 signaling in sustaining chronic inflammation (A) and driving fibrosis (B) can be inhibited by belumosudil (C), thereby rebalancing the immune response.
αSMA, alpha smooth muscle actin; Bcl6, B-cell lymphoma 6; CTGF, connective tissue growth factor; Foxp3, forkhead box P3; IL, interleukin; IRAF4, interferon regulatory factor 4; JAK, Janus-associated kinase; LPA, lysophosphatidic acid; MRTF, myocardin-related transcription factor; ROCK2, rho-associated coiled coil-containing protein kinase-2; RORγT, retinoic acid receptor-related orphan nuclear receptor γ; STAT, signal transducer and activator of transcription; Tfh, follicular helper T cell; TGFβ, transforming growth factor β; Th17, type 17 helper T cell; Treg, regulatory T cell.
*Adapted from Zanin-Zhorov and Blazar.2
Belumosudil is an oral selective ROCK2 inhibitor that has been investigated in the phase II ROCKstar trial (NCT03640481). This trial evaluated the safety and efficacy of 200 mg belumosudil (KD025) once daily versus twice daily for patients with cGvHD who had received two to five prior lines of treatment. The 14-month data were recently published by Cutler and colleagues in the journal Blood1 and are summarized below.
Study design and baseline charactersitics1
The phase II, randomized, multicenter trial evaluating the safety and efficacy of oral, belumosudil 200 mg once daily (n = 66) or twice daily (n = 66) for the treatment of patients who were ≥12 years old, had active cGvHD, and had received two to five prior lines of systemic cGvHD therapy.
- Primary endpoint: overall response rate (ORR) (per 2014 National Institutes of Health criteria).
- Secondary endpoints: safety, duration of response, corticosteroid dose reduction, failure-free survival, changes in Lee symptom score, and overall survival.
The median age across both arms was 56 years, and the baseline characteristics were well balanced (as seen in Table 1).
Table 1. Baseline characteristics*
|
BID, twice daily; cGvHD, chronic graft-versus-host disease; QD, once daily. |
||
|
Belumosudil 200 mg |
||
|---|---|---|
|
|
QD (n = 66) |
BID (n = 66) |
|
Median age, years (range) |
53 (21–77) |
57 (21–77) |
|
Male, % |
64 |
50 |
|
Median prior lines of treatment, n |
3 |
4 |
|
Median time from cGvHD, months |
25 |
30 |
|
Severe cGvHD, % |
70 |
65 |
|
Prior ibrutinib, % |
33 |
35 |
|
Prior ruxolitinib, % |
30 |
27 |
|
≥4 organs involved, % |
50 |
53 |
|
Refractory to prior line, % (n/N) |
79 (44/56) |
65 (35/54) |
Results1
Efficacy
The median duration of response was 54 weeks with responses being maintained for ≥20 weeks in more than half of the patients (59%).
- The primary endpoint of achieving an ORR was 76% in the total population. The efficacy was maintained irrespective of prior treatments (Table 2).
- Complete response was seen in all organ systems (Figure 2), including in organs with fibrotic disease, and the responses were consistent across key subgroups.
- Failure-free survival was seen in 56% of patients at 1 year, and 61% of patients experienced a clinically meaningful improvement (≥7-point reduction) in Lee symptom score.
The median duration of response was 54 weeks in the responder population. Nearly half of the patients (44%) remained on belumosudil for >1 year. In total, 21% of the patients discontinued corticosteroids, while dose reductions of corticosteroids were achieved in 65% of patients.
Table 2. Responses across all subgroups*
|
BID, twice daily; cGvHD, chronic graft-versus-host disease; CI, confidence interval; ORR, overall response rate; QD, once daily. |
|
|
Subgroups |
ORR, % (95% CI) |
|---|---|
|
All patients (N = 132) |
76 (68–83) |
|
Belumosudil 200 mg QD |
74 (62–84) |
|
Belumosudil 200 mg BID |
77 (65–87) |
|
Severe GvHD at screening |
|
|
Yes (n = 89) |
75 (65–84) |
|
No (n = 43) |
77 (61–88) |
|
Best response to last prior line of systemic therapy |
|
|
Refractory (n = 79) |
75 (64–84) |
|
Nonrefractory (n = 31) |
74 (55–88) |
|
Duration of cGvHD before enrollment |
|
|
>50th percentile (n = 66) |
68 (56–79) |
|
≤50th percentile (n = 66) |
83 (72–91) |
|
Number of organs involved at baseline |
|
|
≥4 (n = 68) |
72 (60–82) |
|
<4 (n = 64) |
80 (68–89) |
|
No. of prior lines of systemic therapy |
|
|
≥4 (n = 65) |
74 (61–84) |
|
<4 (n = 67) |
78 (66–87) |
|
Prior ibrutinib (n = 46) |
74 (59–86) |
|
Prior ruxolitinib (n = 38) |
68 (51–82) |
Figure 2. ORR by organ system*
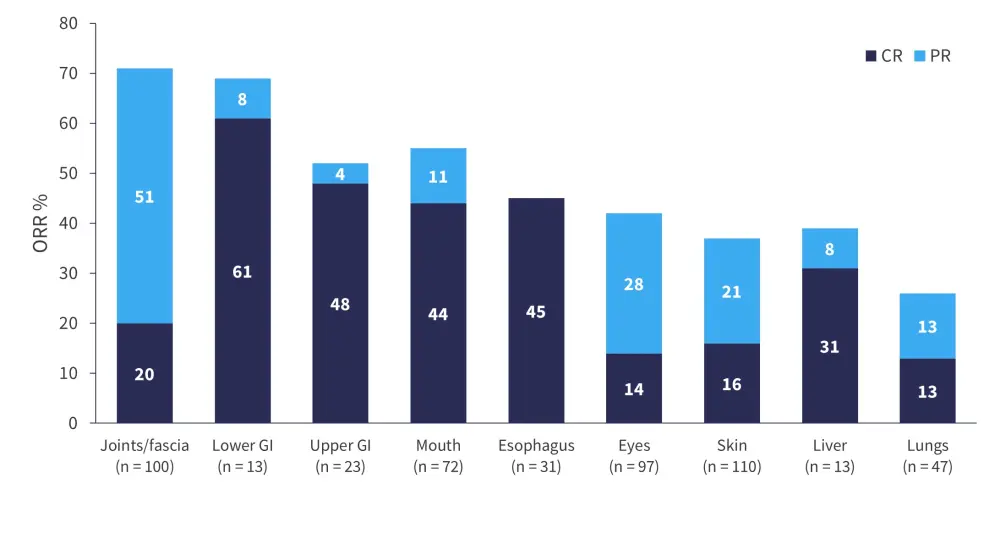
CR, complete response; GI, gastrointestinal; ORR, overall response rate; PR, partial response.
*Data from Cutler et al.1
Safety1
Adverse events (AEs) reported in >20% patients in both arms included fatigue (38%), diarrhea (33%), nausea (31%), cough (28%), upper respiratory infections (27%), dyspnea (25%), headache (24%), peripheral edema (23%), vomiting (21%), and muscle spasms (20%). A minimum of one serious AE was observed in 38% of patients (Table 3). There were a total 14 deaths during the study, of which six occurred during the long-term follow-up (>28 days after the last dose). Overall, both once daily and twice daily belumosudil was well tolerated in patients.
Table 3. Safety*
|
AE, adverse event; BID, twice daily; QD, once daily; SAE, serious adverse event. |
|||
|
|
Belumosudil 200 mg |
|
|
|---|---|---|---|
|
|
QD (n = 66) |
BID (n = 66) |
Total (N = 132) |
|
Median duration of treatment, months |
9.4 |
11.8 |
10 |
|
Any AE, % |
99 |
100 |
99 |
|
Grade ≥3 AE, % |
56 |
52 |
54 |
|
SAE, % |
41 |
35 |
38 |
|
Drug-related AE, % |
74 |
61 |
67 |
|
Drug-related SAE, % |
8 |
3 |
5 |
|
Deaths, % |
12 |
9 |
11 |
Conclusion1
High ORRs were demonstrated in both once daily and twice daily belumosudil arms, meeting the primary endpoint as per the 2014 National Institutes of Health criteria. The responses observed were durable and clinically meaningful, irrespective of organ involvement, patient, and cGvHD characteristics. Despite slightly higher responses in certain organs with the 200 mg twice daily dose, and slightly fewer AEs, 200 mg once daily belumosudil was highlighted as the preferred dosage for the treatment of steroid-refractory cGvHD as the differences between the doses were not significant.
A limitation to the study was that there was no control group; belumosudil was not compared to best available therapy. However, the authors state that it was not deemed appropriate as subjects had previously progressed following at least two systemic lines of therapy, where response rates were historically low and therefore had already tried and failed the best available therapies.
Based on the encouraging efficacy and safety profile demonstrated by this trial, belumosudil was granted approval by the U.S. Food and Drug Administration (FDA) for the treatment of cGvHD in patients ≥12 years of age and who have failed ≥2 prior lines of systemic therapy.
The failure-free survival of patients treated with belumosudil was also recently discussed by Aleksandr Lazaryan during the 63rd ASH Annual Meeting and Exposition. Watch his interview below.
How is failure-free survival impacted by belumosudil in cGvHD which has failed prior therapy?
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

