All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional.
The gvhd Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the gvhd Hub cannot guarantee the accuracy of translated content. The gvhd and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The GvHD Hub is an independent medical education platform, sponsored by Medac and supported through grants from Sanofi and Therakos. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View GvHD content recommended for you
Patterns of liver injury and cytokine profiles in chronic hepatic GvHD after transplant: Refining current NIH consensus criteria
Introduction
Chronic graft-versus-host disease (cGvHD) following allogeneic hematopoietic stem cell transplantation is a multi-organ condition with typical and atypical features. Immune dysregulation and immunodeficiency, driven by both autoimmune and alloimmune processes, lead to impaired organ function and end organ failure; this drives disease comorbidity and ultimately increases mortality.1
Hepatic cGvHD can be misdiagnosed as elevations in liver enzymes and bilirubin can be nonspecific, rising because of other posttransplant hepatobiliary challenges.1 Overdiagnosis can lead to unnecessary immunosuppressive treatments and adverse events, such as opportunistic infections.1
Published in Transplant and Cellular Therapy in 2022, Yang, et al.1 conducted a cross-sectional study (NCT00092235) to characterize liver dysfunction in cGvHD. The study investigated patterns of liver injury as assessed by histopathological findings, liver function tests, clinical data, and immune profiles to evaluate the accuracy of the National Institute of Health (NIH) consensus criteria2 and further elucidate predictors of hepatic cGvHD. Below, we summarize the key findings.
Study design
This was a cross-sectional, prospective multicenter trial recruiting patients through National Cancer Institute (NCI)-designated cGvHD centers. A diagnosis of hepatic cGvHD was made if alanine transaminase (ALT) or total bilirubin was >3 times the upper limit of normal, with no other clinical or biological explanation. cGvHD was scored as mild, moderate, or severe, according to NIH consensus criteria and assessment of the skin, eyes, mouth, liver, gastrointestinal tract, lungs, joints-fascia, and female genital tract (Figure 1). Prospective data collection included clinical and laboratory investigations, histopathology data from liver biopsy, and treatment information, such as immunosuppression intensity (Figure 1)
Figure 1. NIH global cGvHD score assessment and scoring of immunosuppression intensity as used in the cross-sectional study design*
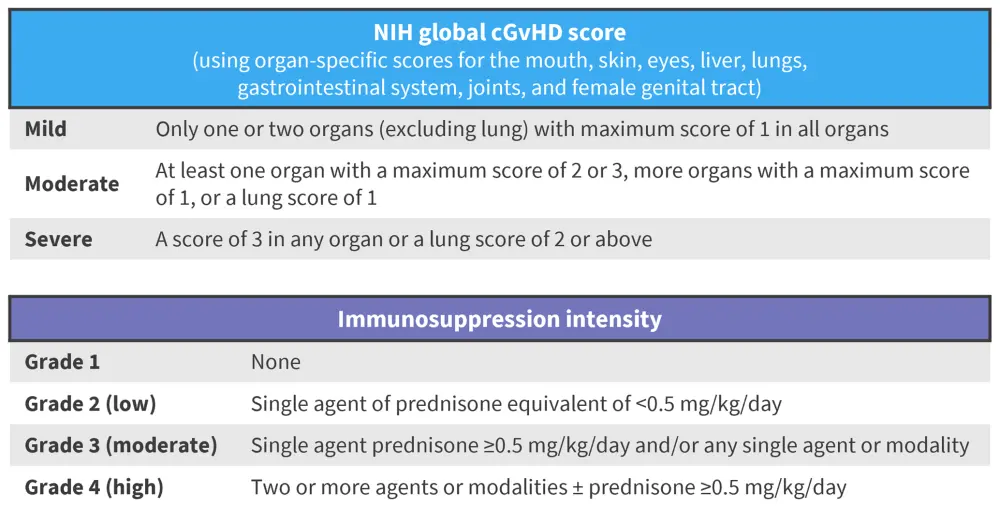
cGvHD, chronic graft-versus-host disease; NIH, National Institute for Health.
*Data from Yang, et al.1
Objectives
- Identify clinical and biological prognostic markers to incorporate into the NIH consensus criteria
Inclusion criteria3
- Aged ≥1 years
- Referred by primary transplant physician for assessment of cGvHD
Exclusion criteria3
- Other significant medical condition
- Pregnancy
- Life expectancy <3 months
Results
Overall, 302 patients were enrolled in the study; the baseline demographic and clinical features can be seen in Table 1. Most patients had undergone hematopoietic stem cell transplantation for hematologic malignancy, with over half (55%) receiving myeloablative conditioning. Of these patients, 55% underwent transplant from HLA-matched related donors. In total, 77% of patients received peripheral blood stem cell grafts. Most patients were receiving moderate to high-intensity immunosuppression at the time of their visit.
Table 1. Baseline demographic, clinical, and treatment characteristics of study participants*
|
BMI, body mass index; cGvHD chronic graft-versus-host disease; IQR, interquartile range; NIH National Institute of Health. |
|
|
Characteristic |
Study participants |
|---|---|
|
Median age at transplant, years (IQR) |
41.5 (28.1–51.9) |
|
Median age at GvHD, years (IQR) |
42.4 (29.3–53.0) |
|
Median age at entry, years (IQR) |
45.4 (31.8–55.9) |
|
Female, n (%) |
128 (42.4) |
|
Median BMI (IQR) |
23.8 (20.5–27.1) |
|
Time from transplant to cGvHD diagnosis, days (IQR), days |
217 (141–367) |
|
Underlying disease, n (%) |
|
|
Hematologic malignancy |
257 (85) |
|
Non-malignant hematologic disease |
30 (10) |
|
Non-hematologic malignancy |
4 (1.4) |
|
Non-malignant disorder |
11 (3.6) |
|
Conditioning regimen, n (%) |
|
|
Myeloablative |
165 (55) |
|
Non-myeloablative/reduced intensity |
137 (45) |
|
Total body irradiation |
111 (37) |
|
NIH consensus criteria, n (%) |
|
|
Mild |
4 (1) |
|
Moderate |
85 (28) |
|
Severe |
210 (70) |
|
Total Score |
8 (5–11) |
|
Organs involved |
|
|
Median number of organs involved (IQR) |
5 (3–6) |
|
Liver, n (%) |
148 (50) |
|
Gastrointestinal tract, n (%) |
135 (45) |
|
Eyes, n (%) |
231 (77) |
Association of platelet count and cytokine levels with hepatic cGvHD
The median platelet count in this cohort was 39 U/ml (interquartile range [IQR], 27–71 U/ml). Using a platelet cutoff of 150 × 103 cells, lower platelet count was significantly associated with higher ALT, total bilirubin, increased portal vein diameters, and lower albumin. An association between liver disease and portal hypertension is suggested by a concurrent increase in portal vein diameter, ALT, and bilirubin in some patients. Several cytokines were associated with either hepatocellular or cholestatic pattern liver injury (Table 2).
Table 2. Cytokines associated with liver injury*
|
IFN, interferon; IL, interleukin. |
|
|
Affected organ |
Cytokine |
|---|---|
|
Hepatocellular injury† |
Serum amyloid A |
|
Cholestatic injury‡ |
IFN-g |
cGvHD diagnosed on consensus criteria
In this study, 148 patients had hepatic cGvHD based on the National Consensus Criteria cGvHD consensus criteria (Table 3). Skin, eyes, and lungs were the most commonly affected organs (79%, 77%, and 76% of patients, respectively). According to the NIH cGvHD consensus criteria, the median total score was 8 (IQR, 5−11), with cGvHD graded severe, moderate, and mild in 70%, 28, and 1% of patients, respectively. A total of 50% of the patients had hepatic involvement of their cGvHD according to NIH consensus criteria, with a median of four other organs involved.
Table 3. The number of patients with an NIH consensus criteria hepatic score of 0–3*
|
cGvHD, chronic graft-versus-host disease; NIH, National Institute of Health. |
|
|
NIH consensus criteria hepatic cGvHD score |
Number of patients |
|---|---|
|
0 |
150 |
|
1 |
99 |
|
2 |
47 |
|
3 |
2 |
GvHD diagnosed on liver biopsy
Liver biopsy was performed in 32 patients. Hepatic cGvHD was identified in 16 patients (59%) on histology, of which six had isolated hepatic cGvHD compared to ten with other liver diseases (Table 4), including steatosis, iron overload, sinusoidal obstructive syndrome, and possible drug-induced liver injury. Only 50% of patients with clinically suspected hepatic cGvHD had histological confirmation. Clinical diagnosis of hepatic cGvHD confirmed on histology had low sensitivity, specificity, positive and negative predictive values of 50%, 27.3%, 50%, and 27.3%, respectively (Kappa, −0.23; 95% confidence interval, −0.59 to 0.13). Demographic and clinical features of patients with and without histologically confirmed hepatic cGvHD were well matched. However, total cholesterol (76% vs 56%; p=0.024), and low-density lipoprotein (LDL) (63% vs 41%; p = 0.030) demonstrated a statistically higher prevalence in patients with cGvHD compared to those without.
Of the 27 biopsies taken, 26 were subject to univariate analysis identifying significant hepatic cGvHD association with higher ALP, total cholesterol, and LDL. Overall, 69% of patients had at least one abnormal liver function test. Multivariate logistic regression identified a significant association with ALP and total cholesterol (Table 4). Using total cholesterol in the diagnostic criteria with a cutoff of 220 mg/dL, the sensitivity, specificity, positive predictive value, and negative predictive value were improved to 75%, 25%, 60%, and 33.3%, respectively.
Table 4. Associations of biopsy-proven hepatic cGvHD versus non-hepatic cGvHD*
|
ALP, alanine phosphatase; LCI, lower confidence interval; LDL, low density lipoprotein; OR, odds ratio; ROC, receiver operative curve; UCI, upper confidence interval. |
|||||||
|
|
Non-hepatic cGvHD |
Biopsy-proven hepatic cGvHD |
p value from t‑test |
Multivariable logistic regression† |
|||
|---|---|---|---|---|---|---|---|
|
OR |
LCI |
UCI |
p value |
||||
|
Total cholesterol |
191 (56) |
258 (76) |
0.024 |
1.26 |
1.02 |
1.56 |
0.035 |
|
LDL |
93 (41) |
145 (63) |
0.030 |
— |
— |
— |
— |
|
ALP |
107 (56) |
199 (113) |
0.012 |
1.16 |
1.01 |
1.33 |
0.040 |
Study limitations
The authors identified several study limitations, including the cross-sectional design, which prevents longitudinal assessment of laboratory parameters. In addition, active treatment of patients may have masked hepatic cGvHD histopathological features or caused confounding drug-related events, such as hypercholesterolemia. Finally, there may have been an introduction of bias due to the physician rather than protocol-guided selection of patients for liver biopsy.
Conclusion
In conclusion, abnormal liver enzymes in cGvHD are non-specific and have poor correlation with histologic evidence for hepatic GvHD, highlighting the importance of histology. Cytokines provide an insight into the pathogenesis of hepatic cGvHD. Decreased platelet count was associated with factors associated with liver disease, including portal vein diameter, which may suggest progression of liver disease. This highlights the need of incorporating these factors into natural history studies and utilizing liver biopsy to understand the development of liver dysfunction in hematopoietic stem cell transplant, to develop better diagnostic instruments, and decrease hepatic cGvHD-related morbidity and mortality.
The authors conclude that while liver function tests are commonly elevated post-HSCT, they are often non-specific to hepatic cGvHD, and that the diagnosis of hepatic cGvHD is hard due to the insensitivity of current diagnostic criteria. Histologic findings must remain the gold standard to confirm diagnosis, with the incorporation of total cholesterol to improve the sensitivity and specificity of the criteria. The association of decreased platelet count with factors associated with liver disease, including portal vein diameter, demonstrated its potential utility as a surrogate marker for the progression of liver disease.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
Which consideration most strongly guides your decision to escalate therapy in SR-aGvHD?

