All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional.
The gvhd Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the gvhd Hub cannot guarantee the accuracy of translated content. The gvhd and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The GvHD Hub is an independent medical education platform, sponsored by Medac and supported through grants from Sanofi and Therakos. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View GvHD content recommended for you
Nutritional interventions in acute GvHD
Do you know... Which of the following is currently not understood to be influenced by nutritional support?
A major trigger of graft-versus-host-disease (GvHD) is intestinal damage induced by preconditioning/conditioning chemotherapy regimens used prior to allogenic hematopoietic stem cell transplantation (allo-HSCT).
This damage to the gut can lead to intestinal failure, malnutrition, escalation of immunosuppressive therapy that promotes sepsis, and potential death if uncontrolled. Treatment strategies implemented in the months before allo-HSCT, performed to control underlying diseases can heighten these effects. Here, we summarize a review by Seguy and Hueso published in Current Opinion on the management of symptoms of malnutrition before and during GvHD.
Nutritional status impact before diagnosis
Role of nutritional status
Treatment strategies implemented pre-allo-HSCT, such as induction and consolidative chemotherapies, prompt a range of side effects, including a decrease in short-chain fatty acids as well as a loss of microbiota diversity. Treatment strategies can also:
- Impact short-term clinical outcomes and survival after allo-HSCT;
- Be highly toxic to intestinal epithelium turnover and damage gut microbiota; and
- Increase the risk of acute GvHD.
Therefore, the composition of oral and gut microbiota can influence the severity and occurrence of GvHD. The status of gut health can be determined through biological markers, such as serum albumin, to indicate the level of inflammatory response and protein catabolism. A multivariate analysis found low levels of albumin (<30 g/dl before allo-HSCT) were associated with:
- 100-day non-relapse mortality in adults;
- Increased risk of developing severe (Grade 3–4) GvHD in the pediatric population;
- In addition, ≥10% weight loss and a body mass index score <5th percentile, increased the risk further.
The evidence supports the use of albumin as a biological marker to indicate the intensity of the inflammatory response as well as protein catabolism for patients pre-allo-HSCT treatment.
Role of gut health status
Plasma citrulline, which is exclusively produced by enterocytes located in the small intestine, can be used to measure intestinal health status in addition to albumin. A retrospective study found a low plasma level of citrulline before allo-HSCT to be associated with a higher risk for Grade 2-4 GvHD. The study concluded that a citrulline level of ≤26ⴗmol/L one month before initiation of conditioning chemotherapy is an independent factor of gastrointestinal GvHD development and non-relapse mortality.
- Dysbiosis and a decrease in citrulline may persist until the allo-HSCT procedure therefore, increasing bacterial translocation, host antigen-presenting cell activation, and severe GvHD.
- Surrogate markers of intestinal barrier disruption were assessed but did not correlate with the lactulose-mannitol ratio.
Prior to allo-HSCT, patients should strengthen their gut barrier through healthy nutrition, and post-allo-HSCT, they should receive early first-line enteral nutrition in order to avoid developing GvHD.
Use of probiotics, prebiotics, and vitamins
Probiotics have also been investigated as another approach to modulating gut microbiota and mitigating GvHD. However, the benefits and timing schedule of probiotic supplementation during allo-HSCT are yet to be determined. The risk of bacterial translocation is highest immediately after chemotherapy treatment; therefore, it would not be beneficial to administer probiotics at this time. However, there have been promising results from fecal microbiota transplants, covered previously on the GvHD Hub, to treat GvHD post-diagnosis, as commensal microbiota will have been replenished.
Prebiotics may be safer to recommend as galacto-oligosaccharides are associated with an increase in short-chain fatty acid and butyrate production. In a murine model, this reduced the severity of GvHD. However, other prebiotics, such as lactose, have shown negative impacts on microbiota diversity, which could in turn enhance GvHD. This suggests not all prebiotics may be advantageous, and further study is required in this area.
While the use of probiotics and prebiotics remains a subject for debate, vitamin D is strongly recommended to be administered before and after allo-HSCT. Evidence from clinical studies has shown:
- A low vitamin D level (<20 ng/ml) before allo-HSCT was associated with a higher incidence of GvHD in pediatric patients.
- Patients treated with ultra-high vitamin D supplementation had a lower GvHD incidence.
- High levels of vitamin D may down-regulate T-cell proliferation, and deficiency may induce high production of pro-inflammatory cytokines.
Nutritional status impact after diagnosis
Enteral versus parenteral nutrition
The Society of Parenteral and Enteral Nutrition recommends oral and enteral nutrition to strengthen the intestinal epithelial barrier before and after allo-HSCT (Figure 1). For acute skin and liver GvHD, parenteral nutrition is not required, and severity of GvHD does not affect this; therefore, oral, and enteral nutrition is recommended as a continuation in order to prevent any further intestinal damage.
On the other hand, in gastrointestinal GvHD, the Society of Parenteral and Enteral Nutrition suggests the use of parenteral nutrition to avoid malnutrition. Once an enterostomy has been performed, oral feeding is encouraged, as this patient cohort is at high risk of death from sepsis and malnutrition. A personalized parenteral nutrition plan may also be required to restore digestion.
Figure 1. Comparison of enteral and oral nutrition versus parenteral nutrition*
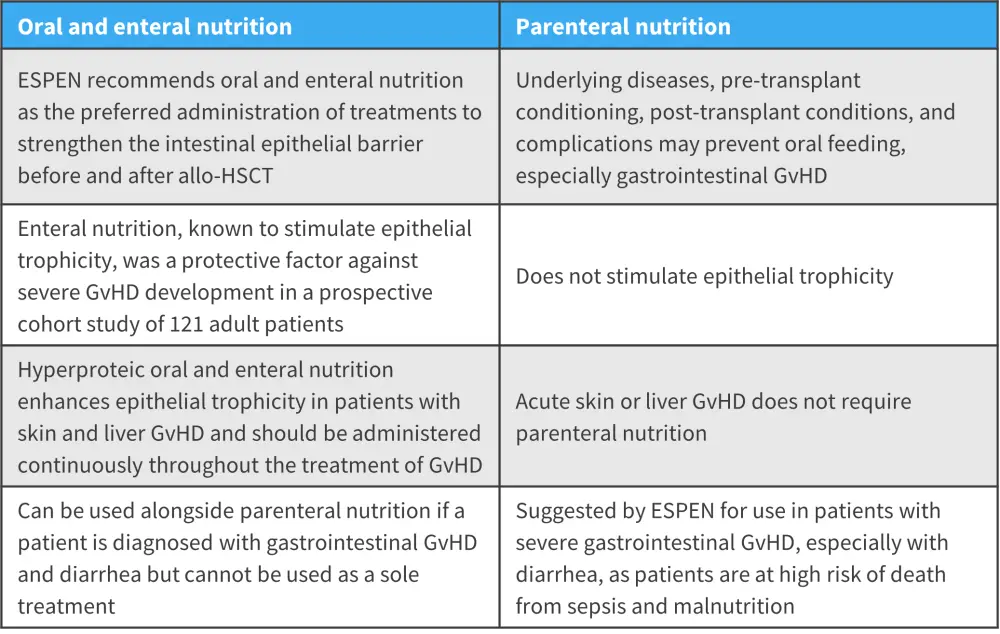
allo-HSCT, allogeneic hematopoietic stem cell transplantation; ESPEN, The Society of Parenteral and Enteral Nutrition; GvHD, graft-versus-host-disease.
*Adapted from Seguy and Hueso1
Conclusion
Strengthening the gut barrier in patients who will receive allo-HSCT through the use of oral and/or enteral nutrition can help to manage the occurrence and severity of acute GvHD. Potential new oral and/or enteral formulas that are specific to reinforcing the intestinal barrier could provide further support to patients with acute GvHD.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
Which consideration most strongly guides your decision to escalate therapy in SR-aGvHD?


