All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional.
The gvhd Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the gvhd Hub cannot guarantee the accuracy of translated content. The gvhd and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The GvHD Hub is an independent medical education platform, sponsored by Medac and supported through grants from Sanofi and Therakos. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View GvHD content recommended for you
High- vs low-dose antithymocyte globulin for pediatric patients with leukemia undergoing allo-PBSCT
Allogeneic hematopoietic stem cell transplantation (allo-HSCT) has improved the survival outcomes of children with leukemia. Antithymocyte globulin (ATG) and anti-T lymphocyte globulin (ATLG) have been shown to reduce the risk and severity of acute and chronic graft-versus-host disease (GvHD) following allo-HSCT, thereby improving non-relapse mortality.
ATG and ATLG represent two distinct GvHD prophylaxis formulations that cannot be administered interchangeably. In pediatric patients, a dose of 15 mg/kg ATLG for myeloablative conditioning in sibling and unrelated transplants has been recommended internationally. However, there is insufficient data to support a uniform dose of ATG in pediatric patients undergoing allo-HSCT.
A study by Hyun Mi Kang et al.1 investigated the effects of different ATG dosages on the overall survival (OS), relapse outcomes, and infectious complications in pediatric patients undergoing allogeneic peripheral blood stem cell transplant (allo-PBSCT) for the treatment of leukemia.
Study design
- Retrospective cohort study of patients aged < 18 years who received HSCT at Seoul St. Mary’s Hospital Pediatric Hemato-oncology Center, KR, between April 2009 and September 2018 (Figure 1).
- High-dose ATG was defined as 7.5 mg/kg (unrelated donor) and 10.0 mg/kg (haploidentical donor), while low-dose ATG was 3.75 mg/kg (unrelated donor) and 5.0 mg/kg (haploidentical donor).
- Primary outcomes: OS and relapse rate.
- Secondary outcomes: acute and chronic GvHD severity and infectious complications due to delayed immune activation following allo-PBSCT.
Figure 1. Study cohort selection1
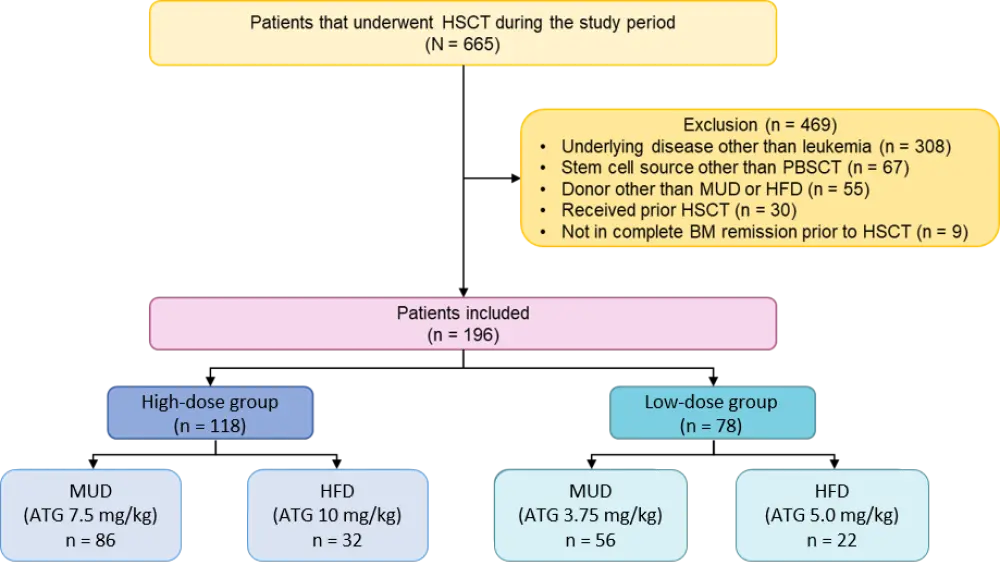
ATG, antithymocyte globulin; BM, bone marrow; HFD, haploidentical familial donor; HSCT, hematopoietic stem cell transplant; MUD, matched unrelated donor; PBSCT, peripheral blood stem cell transplant.
Results
Patient characteristics
Throughout the study period, 196 patients met the eligibility criteria, and the baseline patient characteristics are shown in Table 1.
Table 1. Patient characteristics1
|
Characteristic |
ATG dose group |
p value |
|
|
Low (n = 78) |
High (n = 118) |
||
|
ATG, antithymocyte globulin; CMV, cytomegalovirus; D, donor; EBMT, European Society for Blood and Marrow Transplantation; IQR, interquartile range; R, recipient. Significant differences between groups are shown in bold. *Lymphoid: acute lymphoblastic leukemia, mixed-phenotype acute leukemia; myeloid: acute myeloid, chronic myeloid, juvenile myelomonocytic leukemia. |
|||
|
Male sex, % |
65.4 |
61.0 |
0.536 |
|
Median age at transplant, years (IQR) |
7.7 (5.0–13.6) |
10.5 (3.6–14.7) |
0.659 |
|
Median follow-up duration after transplant, years (IQR) |
2.2 (1.2–3.7) |
5.0 (0.5–7.4) |
0.008 |
|
Type of leukemia, %* |
|
|
0.415 |
|
Lymphoid |
50.0 |
44.1 |
|
|
Myeloid |
50.0 |
55.9 |
|
|
Donor type, % |
|
|
0.976 |
|
Haploidentical familial donor |
26.9 |
27.1 |
|
|
Matched unrelated donor |
73.1 |
72.9 |
|
|
EBMT disease stage at transplant, % |
|
|
0.259 |
|
Early |
67.9 |
65.3 |
|
|
Intermediate |
32.1 |
31.3 |
|
|
Late |
0.0 |
3.4 |
|
|
CMV serostatus (D/R) |
|
|
0.323 |
|
D+/R+ |
93.6 |
96.6 |
|
|
D−/R+ |
6.4 |
3.4 |
|
ATG dose, survival, and relapse
- Lower ATG doses and earlier EBMT diseas e stages at transplant significantly improved OS (p = 0.017) and relapse-free survival (p = 0.021).
- By contrast, higher ATG doses increased the risk of relapse (p = 0.038) and death (p = 0.036).
- The negative impact of higher ATG doses on relapse incidence was confirmed using multivariate analysis, as demonstrated in Tables 2 and 3.
- Non-relapse mortality did not differ significantly between the low- vs high-dose ATG groups (Table 4).
Table 2. Univariate and multivariate analyses of the factors associated with OS in pediatric patients undergoing allo-PBSCT for the treatment of leukemia1
|
|
OS |
|||
|
Univariate analysis |
Multivariate analysis |
|||
|
ATG, antithymocyte globulin; CI, confidence interval; EBMT, European Society for Blood and Marrow Transplantation; HR, hazard ratio; OS, overall survival. Significant differences between groups are shown in bold. |
||||
|
Factor |
HR (95% CI) |
p value |
HR (95% CI) |
p value |
|
Lymphoid vs myeloid |
0.76 (0.44–1.30) |
0.31 |
|
|
|
Age at transplant |
1.00 (0.98–1.10) |
0.19 |
|
|
|
EBMT disease stage at transplant |
2.70 (1.70–4.30) |
<0.001 |
2.59 (1.66–4.04) |
<0.001 |
|
Donor type |
1.60 (0.89–2.70) |
0.12 |
|
|
|
ATG dose |
0.91 (0.70–1.20) |
0.02 |
2.02 (1.05–3.88) |
0.036 |
|
Conditioning regimen |
0.96 (0.72–1.30) |
0.52 |
|
|
Table 3. Univariate and multivariate analyses of the factors associated with RI in pediatric patients undergoing allo-PBSCT for the treatment of leukemia1
|
|
RI |
|||
|
Univariate analysis |
Multivariate analysis |
|||
|
Factor |
HR (95% CI) |
p value |
HR (95% CI) |
p value |
|
ATG, antithymocyte globulin; CI, confidence interval; EBMT, European Society for Blood and Marrow Transplantation; HR, hazard ratio; RI, relapse incidence. Significant differences between groups are shown in bold. |
||||
|
Lymphoid vs myeloid |
0.79 (0.48–1.30) |
0.35 |
|
|
|
Age at transplant |
1.00 (0.95–1.00) |
0.92 |
|
|
|
EBMT disease stage at transplant |
2.20 (1.50–3.40) |
<0.001 |
2.15 (1.42–3.26) |
<0.001 |
|
Donor type |
0.91 (0.52–1.60) |
0.75 |
|
|
|
ATG dose |
1.90 (1.10–3.30) |
0.023 |
1.81 (1.03–3.17) |
0.038 |
|
Conditioning regimen |
1.0 (0.80–1.30) |
0.87 |
|
|
Table 4. Patient outcomes1
|
Outcome, % |
ATG dose group |
p value |
|
|
Low (n = 78) |
High (n = 118) |
||
|
aGvHD, acute graft-versus-host disease; ATG, antithymocyte globulin; cGvHD, chronic graft-versus-host disease; CI, cumulative incidence; NRM, non-relapse mortality; OS, overall survival; RI, relapse incidence. |
|||
|
2-year OS |
83.3 |
71.4 |
|
|
1-year RI |
18.1 |
32.2 |
|
|
2-year RI |
22.2 |
34.8 |
0.022 |
|
1-year NRM |
2.6 |
5.9 |
0.270 |
|
CI Day 100 aGvHD, % |
59.0 |
51.7 |
0.096 |
|
CI 6-month cGvHD, % |
18.8 |
20.2 |
0.672 |
ATG dose and GvHD
- There was no significant difference in the cumulative incidences of acute GvHD between the high vs low groups (Table 4).
- There was also no significant difference in the cumulative incidences of chronic GvHD between the high vs low groups at 6, 12 or 24 months post-transplant (Table 4). Nor was the severity of cGvHD at 12 months affected by ATG dose.
ATG dose and immune reconstitution
- Reconstitution of T4 cells, T8 cells, NKT cells, and B cells was significantly delayed in the high-dose ATG group at 1-month post-transplant.
ATG dose and infectious complications
- Infectious complications following allo-PBSCT were exacerbated in patients who received high vs low dose ATG, as illustrated in Table 5.
Table 5. Infectious complications1
|
Infectious complication |
ATG dose group |
p value |
|
|
ATG, antithymocyte globulin; CMV, cytomegalovirus; EBV, Epstein-Barr virus; HSCT, hematopoietic stem cell transplantation; IQR, interquartile range. Significant differences between groups are shown in bold. |
|||
|
|
Low (n = 78) |
High (n = 118) |
|
|
CMV DNAemia, % |
51.3 |
70.3 |
0.007 |
|
Cases needing preemptive therapy |
43.6 |
66.9 |
0.001 |
|
Onset after HSCT, median (IQR), days |
26 (20–35.8) |
20 (14–27) |
0.022 |
|
CMV disease, % |
10.3 |
3.4 |
0.097 |
|
EBV DNAemia, % |
39.7 |
81.4 |
< 0.001 |
|
Invasive fungal infection, % |
5.1 |
7.6 |
0.491 |
|
Invasive bacterial infection, % |
0 |
12.7 |
0.001 |
Conclusion
In a cohort of pediatric patients undergoing allo-PBSCT for the treatment of leukemia, those who received low doses (3.75–5.0 mg/kg) of ATG demonstrated superior treatment outcomes when compared with those treated with higher ATG doses (7.5–10.0 mg/kg). Higher doses of ATG were also coupled with a significant increase in the rates of viral and bacterial infections and exhibited no benefit over low-dose ATG in preventing the incidence and severity of GvHD.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
Which consideration most strongly guides your decision to escalate therapy in SR-aGvHD?

