All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional.
The gvhd Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the gvhd Hub cannot guarantee the accuracy of translated content. The gvhd and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The GvHD Hub is an independent medical education platform, sponsored by Medac and supported through grants from Sanofi and Therakos. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View GvHD content recommended for you
Gene polymorphisms in the cyclophosphamide metabolism pathway correlate with complications after haplo-HSCT
Haploidentical hematopoietic stem cell transplantation (haplo-HSCT) is used as curative treatment for patients with hematologic malignancies who do not have a full-matched donor. Posttransplant cyclophosphamide is used as a prophylactic treatment to prevent graft-versus-host disease (GvHD) after haplo-HSCT. Cyclophosphamide is an inactive prodrug that is metabolized by polymorphic enzymes in the liver to produce phosphoramide mustard, a DNA alkylating agent with antineoplastic and immunosuppressive activities.1
During the 47th Annual Meeting of the European Society for Blood and Marrow Transplantation (EBMT), Paula Muñiz, Gregorio Marañón General University Hospital, Madrid, ES, presented a retrospective study aimed at identifying polymorphisms in genes of the cyclophosphamide metabolism pathway that correlate with post-HSCT complications, including GvHD, transplant-related mortality (TRM), sinusoidal obstruction syndrome (SOS), and hemorrhagic cystitis (HC).1
Study design and patient characteristics
182 consecutive patients that had received a haplo-HSCT with posttransplant cyclophosphamide (50mg/kg on Days 3 and 4) at the Gregorio Marañón General University Hospital between 2007–2019 were included in the analysis. Treatment schema for this patient population can be seen in Figure 1. Patients received either myeloablative conditioning or reduced intensity conditioning prior to haplo-HSCT.
Figure 1. Treatment schema*
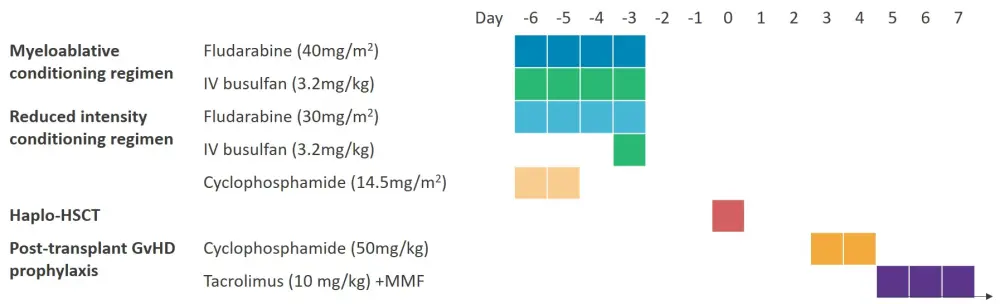
GvHD, graft-versus-host disease; Haplo-HSCT, haploidentical hematopoietic stem cell transplantation; IV, intravenous; MMF, mycophenolate mofetil.
*Adapted from Paula Muñiz et al., 20211
A custom panel of 11 genes relating to cyclophosphamide metabolism were analyzed by genotyping in a MiSeq platform.
- Variations located in the coding and splicing sites were analyzed.
- Polymorphisms that corresponded to a read depth ≥30X in the canonical isoform with an allele frequency greater than 40%, and represented in at least 5% of the cohort, were selected.
Patient characteristics can be seen in Table 1.
- Approximately half of the patients had acute myeloid leukemia or non-Hodgkin lymphoma, and in total, 57% were in complete remission pretransplant.
- Posttransplant complications were: 41% of patients developed Grade II-IV acute GvHD (aGvHD), 35% developed global chronic GvHD (cGvHD), 9% developed SOS, and 25% developed HC.
Table 1. Patient characteristics*
|
aGvHD, acute graft-versus-host disease; ALL, acute lymphocytic leukemia; AML, acute myeloid leukemia; cGvHD, chronic graft-versus-host disease; CLL, chronic lymphocytic leukemia; CML, chronic myeloid leukemia; HC, hemorrhagic cystitis; HL, Hodgkin lymphoma; MDS, myelodysplastic syndrome; MM, multiple myeloma; NHL, non-Hodgkin lymphoma; SOS, sinusoidal obstruction syndrome. |
|
|
Characteristic |
N = 182 |
|---|---|
|
Median age of recipient, years (range) |
48 (16–67) |
|
Median age of donor, years (range) |
40 (14–74) |
|
Sex of recipient, male/female % |
67.03/32.97 |
|
Sex of donor, male/female % |
54.95/45.05 |
|
Diagnosis, % |
|
|
AML |
35.71 |
|
NHL |
13.74 |
|
ALL |
10.44 |
|
MDS |
9.89 |
|
MM |
2.75 |
|
HL |
11.54 |
|
Other (aplastic anemia, CLL, CML) |
15.93 |
|
Pretransplant status, % |
|
|
Active disease/partial response |
43.41 |
|
Complete remission |
56.6 |
|
Conditioning regimen, % |
|
|
Myeloablative |
45.05 |
|
Reduced intensity |
54.95 |
|
Prior transplant, % |
35.16 |
|
Serum ferritin levels >1,648 (µg/mL), % |
50.54 |
|
Busulfan >1.5 days, % |
80.76 |
|
aGvHD, % |
|
|
Grade II–IV |
41.20 |
|
Grade III–IV |
13.19 |
|
cGvHD, % |
|
|
Global |
35.16 |
|
Extensive |
18.68 |
|
SOS, % |
9.34 |
|
HC, % |
24.73 |
Results1
In total, 35 polymorphisms across nine genes were found. The 19 single nucleotide polymorphisms (SNPs) that demonstrated a correlation with post-haplo-HSCT complications by univariate analysis are shown in Table 2.
- SNPs associated with a decrease in activity of cyclophosphamide activation enzymes (cytochrome P450) were associated with a higher incidence of GvHD, TRM, and SOS.
- SNPs associated with a decrease in activity of detoxification enzymes (glutathione S-transferases) were associated with a higher incidence of severe GvHD, TRM, and SOS.
Table 2. Univariate analysis of SNPs associated with posttransplant complications*
|
aGvHD, acute graft-versus-host disease; cGvHD, chronic graft-versus-host disease; HC, hemorrhagic cystitis; Mod-sev, moderate to severe; OR, odds ratio; SNP, single nucleotide polymorphism; SOS, sinusoidal obstruction syndrome; TRM, transplant-related mortality. |
|||||
|
Gene |
SNP |
Modulation of enzyme activity |
Variant effect |
Posttransplant complication, OR (p value) |
|
|---|---|---|---|---|---|
|
Activation |
CYP2A6 |
rs4986892 |
Unknown |
Synonymous |
cGVHD 0.38 (0.02) |
|
rs1801272 |
Loss of function |
Missense |
II–IV aGvHD 2.74 (0.03) |
||
|
rs143731390 |
Decrease |
Missense |
TRM 3.1 (0.01) |
||
|
CYP2B6 |
rs3745274 |
Decrease |
Missense |
Mod-sev cGvHD 0.37 (0.01) |
|
|
rs3211371 |
Decrease |
Missense |
II–IV aGvHD 2.46 (0.01) |
||
|
rs2279341 |
Unknown |
Synonymous |
cGvHD 2.11 (0.03) |
||
|
rs2279343 |
Unknown |
Missense |
HC 3.16 (0.03) |
||
|
rs3745274(wt) |
— |
— |
SOS 1.6 (0.002) |
||
|
CYP2C8 |
rs10509681 |
Decrease |
Missense |
II–IV aGvHD 1.59 (0.01) |
|
|
rs11572080 |
Decrease |
Missense |
II–IV aGvHD 2.41 (0.04) |
||
|
CYP2C9 |
rs1799853 |
Decrease |
Missense |
II–IV aGvHD 1.67 (0.03) |
|
|
CYP2C19 |
rs4244285 |
Decrease |
Synonymous |
TRM 2.45 (0.01) |
|
|
rs3758580 |
Unknown |
Synonymous |
TRM 2.02 (0.04) |
||
|
Detoxification |
GSTA1 |
rs1051775 |
Unknown |
Synonymous |
III–IV aGvHD 0.46 (0.003) |
|
GSTA1*B |
Decrease |
Missense |
III–IV aGvHD 2.53 (0.01) |
||
|
GSTM1 |
rs1065411 |
Unknown |
Missense |
TRM 1.94 (0.03) |
|
|
GTSM1*0 |
Loss of function |
Null allele |
SOS 2.3 (0.04) |
||
|
GSTP1 |
rs1659 |
Decrease |
Missense |
III–IV aGvHD 2.77 (0.04) |
|
|
GSTT1 |
GSTT1*0 |
Loss of function |
Null allele |
III–IV aGvHD 2.62 (0.01) |
|
Multivariate analysis revealed that eight polymorphisms in five genes were associated with posttransplant complications (Table 3).
- SNPs associated with a decrease in activity of activation enzymes, were associated with a higher incidence of GvHD.
- SNPs that were associated with a decrease in activity of detoxification enzymes were associated with a higher incidence of severe GvHD, TRM, and SOS.
Table 3. Multivariate analysis of SNPs associated with posttransplant complications*
|
aGvHD, acute graft-versus-host disease; cGvHD, chronic graft-versus-host disease; HS, hemorrhagic cystitis; Mod-sev, moderate to severe; SHR, subdistribution hazard ratio; SNP, single nucleotide polymorphisms; SOS, sinusoidal obstruction syndrome; TRM, transplant-related mortality. |
|||||
|
Gene |
SNP |
Modulation of enzyme activity |
Variant effect |
Posttransplant complication, SHR (p value) |
|
|---|---|---|---|---|---|
|
Activation |
CYP2A6 |
rs143731390 |
Decrease |
Missense |
Mod-sev cGvHD 3.44 (0.003) |
|
|
CYP2B6 |
rs3745274 |
Decrease |
Missense |
Mod-sev cGvHD 0.38 (0.02) |
|
|
|
rs3211371 |
Decrease |
Missense |
II–IV aGvHD 2.02 (0.008) |
|
Detoxification |
GSTA1 |
rs1051775 |
Unknown |
Synonymous |
III–IV aGvHD 0.42 (0.042) |
|
|
|
GSTA1*B |
Decrease |
Missense |
TRM 2.32 (0.036) |
|
|
GSTM1 |
rs1065411 |
Unknown |
Missense |
TRM 2.13 (0.01) |
|
|
|
GTSM1*0 |
Loss of function |
Null allele |
SOS 1.36 (0.032) |
|
|
GSTT1 |
GSTT1*0 |
Loss of function |
Null allele |
III–IV aGvHD 3.29 (0.005) |
Conclusions
Genetic variation in genes involved in the cyclophosphamide metabolism pathway correlated with several post-haplo-HSCT complications, most notably GvHD, but also TRM, and SOS. Only one SNP correlated with HC in the univariate analysis. Therefore, the analysis of these variants before haplo-HSCT transplant could facilitate personalized risk approaches and clinical management of patients with hematologic malignancies. However, these results need to be validated in other patient cohorts.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
Which consideration most strongly guides your decision to escalate therapy in SR-aGvHD?

