All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional.
The gvhd Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the gvhd Hub cannot guarantee the accuracy of translated content. The gvhd and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The GvHD Hub is an independent medical education platform, sponsored by Medac and supported through grants from Sanofi and Therakos. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View GvHD content recommended for you
Findings from the prospective iMAGINE trial of ibrutinib in pediatric patients with chronic GvHD
Chronic graft-versus-host disease (cGvHD) is a significant cause of mortality and morbidity after allogeneic hematopoietic stem cell transplantation (allo-HSCT) in children, which limits their treatment options. Ibrutinib, a Bruton’s tyrosine kinase inhibitor is approved by the U.S. Food and Drug Administration (FDA) for the treatment of adult patients with cGvHD following failure of previous systemic therapy and has shown positive results in this population.
A supplemental New Drug Application (sNDA) for the treatment of pediatric and adolescent patients with ibrutinib was recently submitted to the U.S. FDA for approval. The submission was based on interim findings from the phase I/II iMAGINE trial (NCT03790332). During the 48th Annual Meeting of the European Society for Bone and Marrow Transplantation (EBMT), Marco Zecca1 presented results from the iMAGINE trial, and the key findings are summarized here.
Study design
This was an open-label, multicenter phase I/II study trial in patients aged ≥1 and <12 years with moderate-to-severe cGvHD who had failed ≥1 line of systemic therapy or patients aged ≥12 and <22 years with moderate-to-severe cGvHD who were newly diagnosed or had failed ≥1 line of systemic therapy. The study design is shown in Figure 1; different dosing schedules of ibrutinib were used depending on whether a patient had treatment-naïve (n = 12) or relapsed/refractory (R/R; n = 47) MM.
Figure 1. Treatment schema*
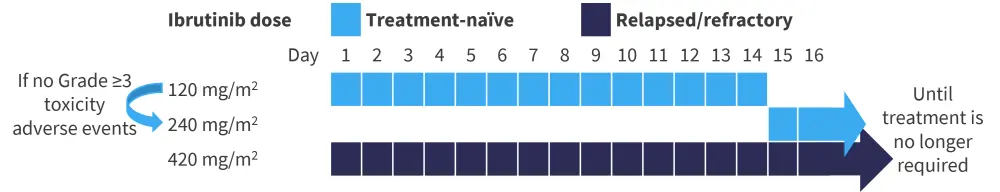
*Adapted from Zecca.1
The primary endpoints included pharmacokinetics and safety. Secondary endpoints included overall response rate according to the National Institute of Health 2014 criteria for cGvHD, overall survival (OS), and duration of response (DoR).
Results
Baseline characteristics
A total of 59 patients were included, with a median age of 13 years (range, 1–19 years), and 71% of all patients were male. Overall, 64% of patients were treated with allo-HSCT for a malignant disease (Table 1). At baseline, skin was the most involved organ (80%), followed by mouth (63%), eyes (58%), and joints and fascia (56%). In patients aged <12 years, 30% had lung involvement, compared with 56% of patients aged ≥12 years.
Table 1. Baseline patient characteristics*
|
cGvHD, chronic graft-versus-host disease; HSCT, hematopoietic stem cell transplant; R/R, relapsed/refractory; N/A, not applicable. |
|||
|
Characteristic |
Treatment-naïve |
R/R |
All patient |
|---|---|---|---|
|
Median age (range), years |
11.5 (3−17) |
13 (1−19) |
13 (1−19) |
|
<12 years, % |
50 |
45 |
46 |
|
≥12 years, % |
50 |
55 |
54 |
|
Male, % |
75 |
70 |
71 |
|
Karnofsky/Lanksy performance status score, % |
|
|
|
|
<80 |
25 |
26 |
25 |
|
≥80 |
75 |
74 |
75 |
|
Median time from allo-HSCT to diagnosis (range), months |
8 (3−13) |
8 (3−50) |
8 (3−50) |
|
Median time from initial cGvHD diagnosis to enrollment (range), months |
1 (0.1−78) |
16 (0.2−163) |
12 (0.1−163) |
|
Median prior cGvHD therapies (range)† |
N/A |
2 (1−12) |
2 (1−12) |
|
Underlying disease indicated for HSCT, % |
|
|
|
|
Malignant |
66 |
64 |
64 |
|
Non-malignant |
33 |
36 |
36 |
The median follow-up was 20 months (range, 2−32 months) and the median duration of treatment was 8 months (range, 0.1−26 months). In the treatment-naïve group, 58% of patients continued ibrutinib, compared with 32% in the R/R group.
Pharmacokinetics
Patients in both groups discontinued treatment, as shown in Figure 2. A higher percentage discontinued ibrutinib treatment in the R/R group (68.1%) compared with in the treatment-naïve group (41.6%).
Figure 2. Consort diagram for all patients included in the study*
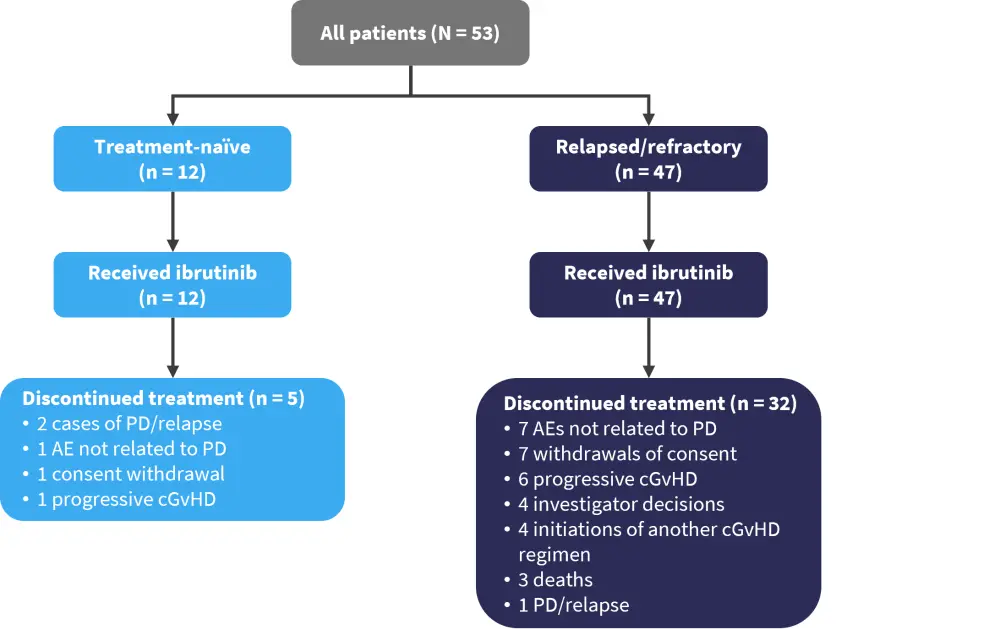
AE, adverse event; cGvHD, chronic graft-versus-host disease; PD, progressive disease.
*Adapted from Zecca.1
The recommended pediatric equivalent dose for patients aged <12 years and ≥12 years was 240 mg/m2 daily and 420 mg/m2 daily, respectively. The plasma concentration-time profile of ibrutinib in both younger and older children was consistent with those of adult patients with cGvHD.
Safety
Treatment-emergent adverse events (TEAEs) were common and occurred in 98% of all patients, with Grade ≥3 TEAEs occurring in 58% and 66% of patients in the treatment-naïve and R/R groups, respectively. Three fatal AEs were reported in the R/R group, compared with none in the treatment-naïve group. Serious AEs occurred in 64% and 67% of patients in the R/R and treatment-naïve groups, respectively.
Pyrexia, diarrhea, and abdominal pain were the most frequently reported Grade 1−2 AEs in all patients. The most common Grade ≥3 AEs were pyrexia, stomatitis, increased alanine aminotransferase, and hypokalemia (Figure 3).
Figure 3. Treatment-emergent adverse events observed in all patients*
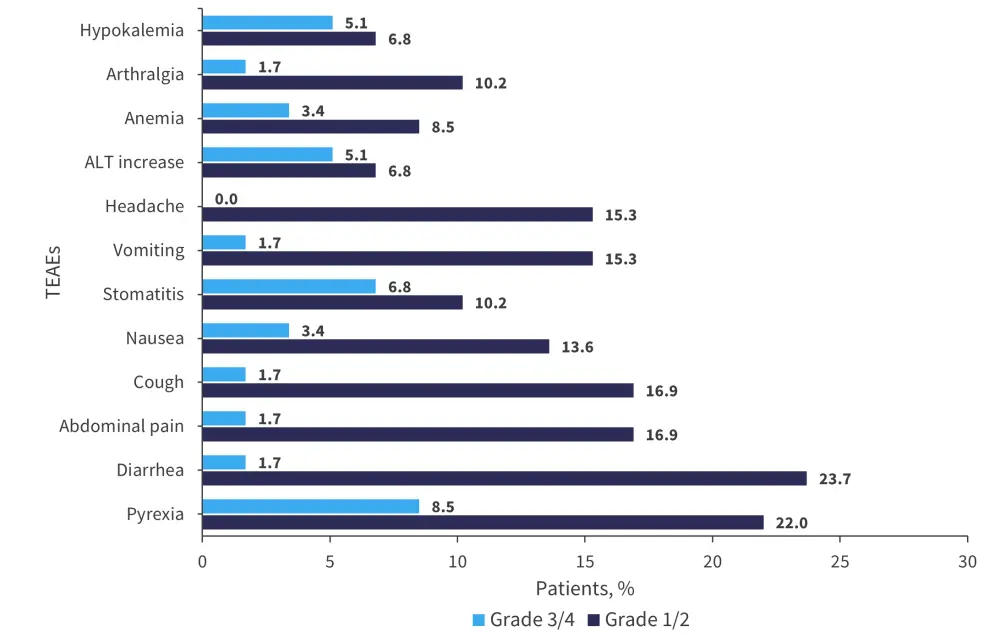
ALT, alanine aminotransferase; TEAEs, treatment-emergent adverse events.
*Adapted from Zecca.1
Efficacy
With a median follow-up of 20 months (range, 2−32 months), OS at 1 year was 92% and 96% in the treatment-naïve and R/R groups, respectively (95% confidence interval [CI], 85–98%). The probability of OS at 1 year was 96% (n = 22) vs 95% (n = 37) in patients with moderate and severe cGvHD.
The overall response rate was 78%, with a median time to first response of 4 weeks (range, 3.7–84.1 weeks) and a median time to best response of 6 weeks (3.7−84.1 weeks). In the treatment-naïve group, 25% of patients achieved a complete response, compared with only 4% in the R/R group (Figure 4).
Figure 4. Response rates in all groups*
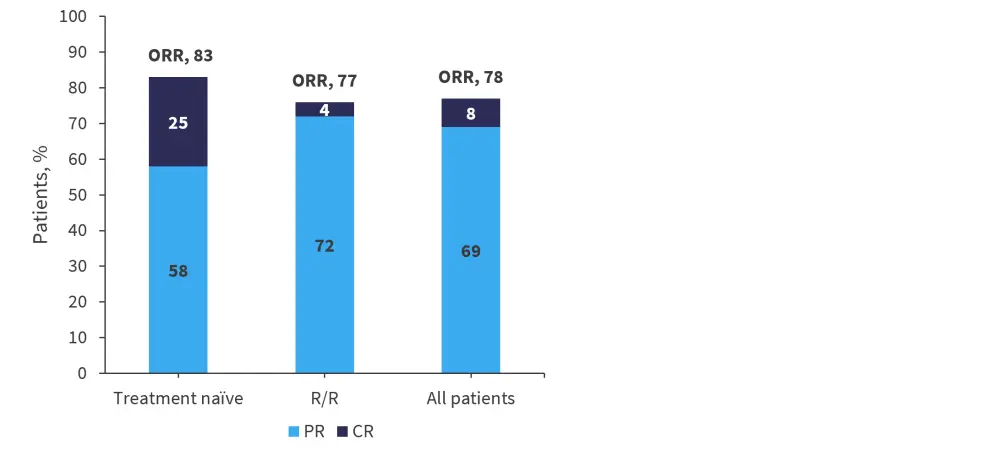
CR, complete response; ORR, overall response rate; PR, partial response; R/R, relapsed/refractory.
*Adapted from Zecca.1
The response rate at 24 weeks was 44%, and 61% of patients sustained the response at ≥20 weeks. With respect to the Lee cGvHD Symptom Scale score, 44% (14/32) of patients aged ≥12 years improved their score by ≥7 points on ≥2 consecutive visits. The median DoR was not reached (95% CI, 8.9−not evaluable) and the estimated DoR at 1 year was 58% (95% CI, 40−73%). Responses were durable and were similar between the treatment-naïve and R/R groups. Although not a prespecified endpoint, organ-specific responses were observed and ranged from 31% in lungs to 90% in upper gastrointestinal tract.
Conclusion
The iMAGINE study demonstrated that the recommended pediatric dose of ibrutinib was 240 mg/m2 in patients aged <12 years and 420 mg/m2 for patients aged ≥12 years and that ibrutinib achieved plasma concentration-time profiles consistent with those observed in adults. A response rate of 78% was achieved with ibrutinib in the whole group, with five patients achieving a complete response. The safety profile was also found to be consistent with the existing profile for ibrutinib in adults with moderate-to-severe cGvHD. Together, these results show that ibrutinib is a viable option for the treatment of pediatric patients with cGvHD.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content


