All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional.
The gvhd Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the gvhd Hub cannot guarantee the accuracy of translated content. The gvhd and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The GvHD Hub is an independent medical education platform, sponsored by Medac and supported through grants from Sanofi and Therakos. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View GvHD content recommended for you
Epigenetic regulation of GvHD pathophysiology
The pathophysiology of graft-versus-host disease (GvHD) evolves in three steps: priming phase, induction phase, and effector phase. Different immune regulatory systems within the host and donor cells operate at each stage. It is hoped that by understanding these pathways better, there is potential for the identification of novel targets for the treatment of acute (a)GvHD.
Epigenetic regulation of gene expression has become a hot topic of study in the last decade and this term refers to determinants of phenotype that are independent of the DNA sequence. Modulation of the immune response to the engrafted cells is in part controlled by epigenetic regulatory systems. This article will summarize the review by Alicia Li and colleagues that was published in Haematologica on this subject.1
Overview
During the priming phase, conditioning regimens, radiation or other toxic factors used prior to infusion of donor cells may cause damage to body cells in recipients, resulting in release of damage-associated molecular patterns and pathogen-associated molecular patterns. These changes lead to the induction phase where released molecules induce antigen presenting cells (APCs) which in turn activate alloreactive infused donor T cells. During the effector phase, the alloreactive cells induce damage to GvHD target organs by cytokine release and direct cytotoxicity. A summary of the interaction of different cells and molecules is shown in Figure 1.
Many processes in the pathophysiology of aGvHD are regulated by different epigenetic players, which are summarized in Table 1 Certain proteins are common across multiple cells/tissues, such as histone deacetylases (HDACs).
Figure 1. Epigenetic regulatory factors during the development of acute graft-versus-host disease1
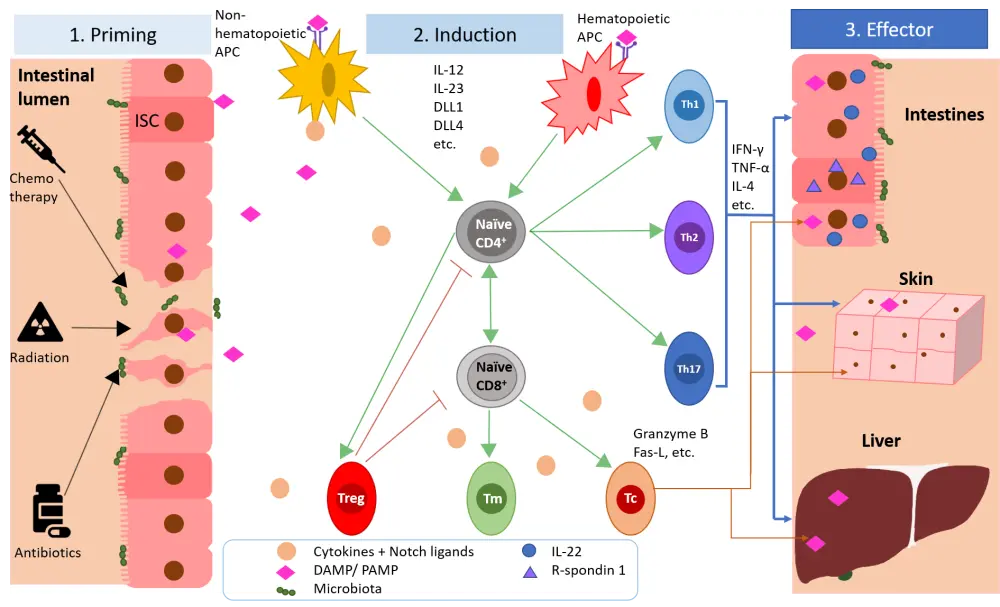
Table 1. Summary of epigenetic regulators in immune cells during acute graft-versus-host disease
|
Epigenetic modulators |
|||
|
APC |
T cell |
Treg |
Intestinal |
|
APC, antigen presenting cell; BMI1, BMI1 proto-oncogene, polycomb ring finger; DNMT, DNA methyltransferases; EZH2, enhancer of zeste homolog 2; HDAC, histone deacetylase; SIRT3, sirtuin 3; Treg, regulatory T cell. |
|||
|
HDAC6 |
DNMT |
DNMT |
BMI1? |
|
HDAC11 |
EZH2 |
EZH2? |
HDAC |
|
|
HDAC1 |
|
|
|
|
HDAC6 |
|
|
|
|
HDAC11 |
|
|
|
|
SIRT3 |
|
|
Epigenetic regulators of APCs
HDACs are responsible for removing acetyl groups from the DNA and cause the chromatin to close up, reducing gene transcription. Pan-HDAC inhibitors, such as suberoylanilide hydroxamic acid (also known as vorinostat) also decrease the production of pro-inflammatory cytokines (e.g., tumor necrosis factor-α, interleukin [IL]-1, and interferon-γ), which promote alloreactive T cell responses and reduce aGvHD in vivo.
This process of APC activation seems to be closely regulated by HDACs. However, different HDACs can play opposite roles in the balance between pro- and anti-inflammatory states. HDAC11 silencing causes increased production of IL-10, an immunosuppressive cytokine. Upon silencing of HDAC6 however, IL-10 expression is repressed. This suggests that the use of specific HDAC inhibitors as opposed to pan-HDAC antagonists may be valuable in shifting the immune response to a more tolerogenic state.
Epigenetic regulators of alloreactive T-cells
As shown in Figure 1, upon activation of donor T cells by host APCs, the T cells undergo expansion and differentiation into alloreactive effector T cells. They can also become memory T cells and may persist for months to years.
Enhancer of zeste homolog 2 (EZH2) acts as a gene silencer by adding methyl groups to histones. EZH2 may also be involved in Th1 and Th2 polarization, along with proliferation and differentiation of hematopoietic stem cells. Ezh2 loss in donor T cells has been shown to repress aGvHD in mice. The T cells in this model could be activated to divide initially but became defective at later stages of aGvHD induction. Silencing Ezh2 or protein inhibition of EZH2 did not compromise the graft-versus-leukemic effect and lead to an increased overall survival in recipients. Therefore, it may be a promising therapeutic target for aGvHD.
Alloantigen-primed memory T cells are thought to sustain the populations of alloreactive effector T cells. Currently these cells seem resistant to available immunosuppressive agents and may be part of the reason for low response rates to traditional therapy. Interestingly, EZH2 is important for multiple stages of memory T cell development and maturation. In CD8+ cells, EZH2 deficiency causes a drastic increase in terminally differentiated cells that can no longer contribute to T cell expansion. In addition, it skews T cell development towards central memory precursors. All this points to a crucial role of EZH2 for maintenance of alloreactive memory T cells during aGvHD and may therefore serve as a worthwhile target for GvHD treatment.
HDAC1 plays a negative regulatory role for induction of Th1 and Th2 cells. Increased induction of these subsets was noted following Hdac1 deletion. HDAC11 is also thought to suppress GvHD as suggested by results obtained with Hdac11-knockout mice showing increased secretion of proinflammatory cytokines and T cell proliferation. By contrast, HDAC6 inhibition can protect against GvHD lesions in transplanted mice and is accompanied by decreased CD8+ effector T cells that secrete interferon-γ and IL-2.
In addition to the nuclear HDACs, mitochondrial HDACs such as SIRT3 exist, which are expressed in alloreactive T cells and other metabolically stressed cells. Evidence suggests that these enzymes may play a role in GvHD as Sirt3 deletion in donor T cells was shown to reduce the severity of GvHD in a mouse model.
DNA methyl transferases (DNMT) can globally suppress transcription. 5-Azacytidine (Aza) can inhibit DNMT and cause impaired T cell activation, proliferation, and cytokine production by decreasing the expression of cell cycle-related genes. In mice undergoing allogeneic hematopoietic stem cell transplantation (allo-HSCT), pretreatment with Aza prevented GvHD without increasing T regulatory cells (Tregs).
Epigenetic regulators of Tregs
Tregs can suppress immune responses both through contact dependent methods and through the production of cytokines. Natural (n)Tregs and inducible (i)Tregs have been most closely studied in the context of GvHD, and an infusion of Tregs has been shown to block lethal GvHD reactions in mice following allo-HSCT. nTregs appear more promising in terms of clinical trial results.
Effector T cells and Tregs have a different expression profile of HDACs, which could be exploited to enhance the suppressive action of Tregs. HDACs 3, 6 and 9 may play a part in controlling Treg function. Inhibition of HDAC6 or 9 expression can lead to increases in forkhead box protein P3 (FOXP3) and lead to enhanced nTreg action.
DNMT inhibitors have been used to stabilize FOXP3 expression and have had some success in reducing aGvHD symptoms. In mouse models, treatments with Aza and another DNMT inhibitor, decitabine, reduced aGvHD and improved survival. However, Foxp3-knockout iTregs maintained their suppressive effects indicating that their aGvHD restraining action may be independent or downstream of FOXP3.
EZH2 plays a role in iTreg identity and its loss impairs differentiation. Murine Ezh2-knockout mice expressed an effector phenotype that may be deleterious in GvHD. Treatment with an anti-EZH2 agent significantly decreased iTregs.
The microbiome may also influence the stimulation and maintenance of Tregs through the production of short chain fatty acids such as butyrate. Lachnospiraceae- and Ruminococcaceae-family bacteria, of the Clostridia class, are correlated with a greater Treg/Th17 ratio and this is associated with increased protection from aGvHD. Butyrate inhibits HDAC activity and treating mice orally with butyrate increases peripheral Tregs. However, in mice deficient in part of the Foxp3 locus, this increase was not seen following butyrate administration. Compared with mice treated with antibiotics and no butyrate, Treg suppressor function was improved in butyrate-treated mice. Naïve CD4+FOXP3- T cells upon treatment with butyrate became activated for 3 days and showed significant increases in acetylation at the Foxp3 promoter. To read more on the role of short chain fatty acids in GvHD click here.
Epigenetic pathways that influence tissue injury and repair
A shown in Figure 1 the effector phase sees effector T cells migrating to specific organs and cytotoxically attacking the cells there. In response to these attacks, the body initiates repair and regeneration pathways in which the intestinal stem cells play a crucial role. However, these cells are frequently attacked by alloreactive T cells, resulting in a nonproductive cycle of damage and repair.
IL-22 protects the intestinal epithelium during GvHD, however the intestinal cells that produce this cytokine are frequently targeted by alloreactive T effector cells leading to IL-22 deficiency. R-spondin1, a Wnt agonist, can aide regeneration by increasing the proliferation of intestinal stem cells. STAT3 is involved in regulating access to the il22 promoter. CD4+ T cells that are STAT3-deficient show decreased production of IL-22 in response to IL-21 stimulation.
The microbiome has recently been shown to contribute to aGvHD. The increased inflammation is damaging to commensal bacteria populations and can lead to enhanced severity of aGvHD in turn. Clostridiale species that produce butyrate decrease during aGVHD. Treatment with exogenous butyrate helps to reverse this, reduces apoptosis of intestinal epithelial cells and improves their junctional integrity. Enhancing the recovery of damaged tissues may represent a potentially beneficial avenue of drug development for treating aGvHD.
Pharmacological modulation of epigenetic pathways in aGvHD
A summary of ongoing clinical trials for epigenetic regulators can be seen in Table 2. In one of the vorinostat trials, an incidence of Grade II-IV GvHD of 22% was seen by Day 100. Treatment with vorinostat showed an acceptable safety profile and was deemed effective. Using panobinostat produced a 40% response rate with corticosteroids and this was seen across patients with Grade III-IV GvHD. However, the study did not have enough power to assess efficacy.
When Aza was used to treat patients following a donor lymphocyte infusion, none of the patients included on the trial developed severe GvHD (≤ Grade III) and there were no aGvHD-related mortalities.
Aza and decitabine, DNMT inhibitors, are frequently used for treating patients undergoing allo-HSCT and for maintenance afterwards, and they can partially alleviate aGvHD. The first trial shown in Table 2 with Aza resulted in no patients dying or developing severe aGvHD. A 3.2-fold increase in Tregs was seen after treatment with a donor lymphocyte infusion and Aza, in the second trial.
So far there has been little success in using EZH2 inhibitors such as GSK126 to reduce aGvHD and prevent the development of effector T cells.
Table 2. Clinical trials of an epigenetic inhibitor in aGvHD1
|
Agent |
Mode of action |
Results |
|
aGvHD, acute graft-versus-host-disease; allo-HSCT, allogeneic hematopoietic stem cell transplantation; Aza, 5-azacytidine; DLI, donor lymphocyte infusion; DNMT, DNA methyltransferase; GvHD, graft-versus-host disease; HDAC, histone deacetylase; SAHA, suberoylanilide hydroxamic acid; Treg, regulatory T cell. |
||
|
Vorinostat/SAHA |
HDAC inhibitor |
With standard prophylaxis, reduced severe aGvHD in a phase I/II trial. Similar results were seen in another phase II trial in patients receiving myeloablative conditioning and methotrexate. |
|
Panobinostat |
HDAC inhibitor |
With glucocorticoids, deemed safe as a primary GvHD therapy. Phase I/II trial. See this interview with Lia Perez for more information. |
|
Aza |
DNMT inhibitor |
Well tolerated after DLI and no patients developed Grade II−IV aGvHD. Phase I trial. See this interview with Frederic Baron for more information. In another phase I/II trial of Aza after allo-HSCT, circulating Tregs were found to have increased. |
Conclusion
While the investigation of the epigenetic regulatory pathway in aGvHD is still a young field, the progress that has been made is very promising. Understanding the basic biology of aGvHD highlights multiple effector molecules that could be targeted to decrease the severity of aGvHD and improve the quality of life of those patients affected. Different angles, such as supportive host regeneration of intestinal epithelial cells or modulating the microbiome, are potential avenues for exploration.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
Which consideration most strongly guides your decision to escalate therapy in SR-aGvHD?

