All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional.
The gvhd Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the gvhd Hub cannot guarantee the accuracy of translated content. The gvhd and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The GvHD Hub is an independent medical education platform, sponsored by Medac and supported through grants from Sanofi and Therakos. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View GvHD content recommended for you
Efficacy of hUCB-MSCs as a salvage therapy for SR-aGvHD
Acute graft-versus-host disease (aGvHD) is a severe complication of allogeneic hematopoietic stem cell transplant, with high rates of morbidity and mortality.1 Steroids are commonly used as a first-line treatment for patients with aGvHD; however, only ~50% of patients respond to this treatment and achieve long-lasting remission. There are currently limited options for patients with steroid-refractory (SR) aGvHD.1
Mesenchymal stem cells (MSCs) have become a prospective cell source for cellular therapies due to their ability to self-renew, with low rates of immunogenicity.1 Previously, clinical trials have reported positive response rates in patients with SR-aGvHD after MSC infusion. However, the majority of these trials have investigated bone marrow-derived MSCs (BM-MSCs), with no large-scale trials utilizing human umbilical cord blood-derived MSCs (hUCB-MSCs). This trial investigated the efficacy of hUCB-MSCs as a salvage therapy in patients with SR-aGvHD.1
Study design1
A total of 54 patients who had developed aGvHD from 2015 to 2019 were included. Of these, 52 and two patients developed aGvHD due to hematopoietic stem cell transplant and donor lymphocyte infusion, respectively. Patients were given hUCB-MSCs at a once-weekly dose of 1.0–2.0 × 106/kg. There were a median of two infusions per patient, with a range of 1–15 infusions.
Results1
Patient characteristics are shown in Table 1. Most patients were male and received haploidentical transplant.
Table 1. Patient characteristics*
|
ATG, antithymocyte globulin; GvHD, graft-versus-host disease; MMF, mycophenolate mofetil; MTX, methotrexate; Tac, tacrolimus. |
|
|
Characteristic, % (unless otherwise stated) |
Total cohort |
|---|---|
|
Median age (range), years |
12.5 (1–62) |
|
Sex |
|
|
Male |
70.3 |
|
Female |
29.6 |
|
Type of transplant |
|
|
Matched unrelated |
3.7 |
|
Matched sibling |
9.2 |
|
Haploidentical |
79.6 |
|
Umbilical cord blood |
7.4 |
|
Graft source |
|
|
Bone marrow |
9.3 |
|
Peripheral blood stem cells |
20.4 |
|
Bone marrow + peripheral blood stem cells |
63 |
|
Umbilical cord blood stem cells |
7.4 |
|
GvHD prophylaxis |
|
|
Cyclosporine + MTX |
5.5 |
|
Cyclosporine/Tac + MTX + MMF |
16.6 |
|
Cyclosporine/Tac + MTX + MMF + ATG |
75.9 |
|
Unknown |
1.9 |
Of the 54 patients included, 48 had classic aGvHD and 6 had late-onset GvHD. Approximately 90% of patients had Grade III or IV GvHD.
Efficacy1
Response rates to hUCB-MSCs was assessed at Day 28 post-treatment initiation. Response rates at Day 28 post-treatment initiation differed for each organ, as shown in Figure 1. aGvHD of the skin showed the highest overall and complete response rates.
Figure 1. Day 28 response rates*
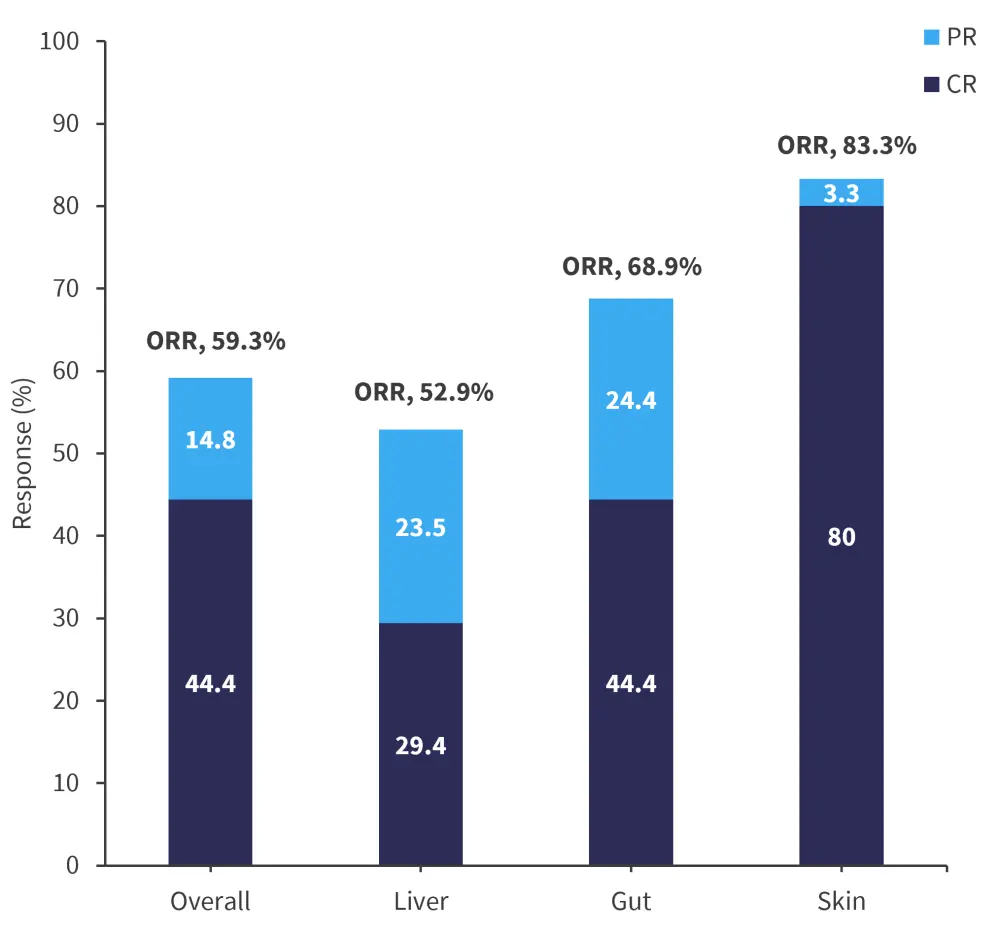
CR, complete response; ORR, overall response rate; PR, partial response.
*Adapted from Ding, et al.1
The median follow-up was 19.3 months, with the longest follow-up being 59 months. Patients who responded to hUCB-MSC treatment had significantly better survival rates than those who did not respond. Survival rates at 1 year are shown in Figure 2.
Figure 2. Survival rates at 1 year*
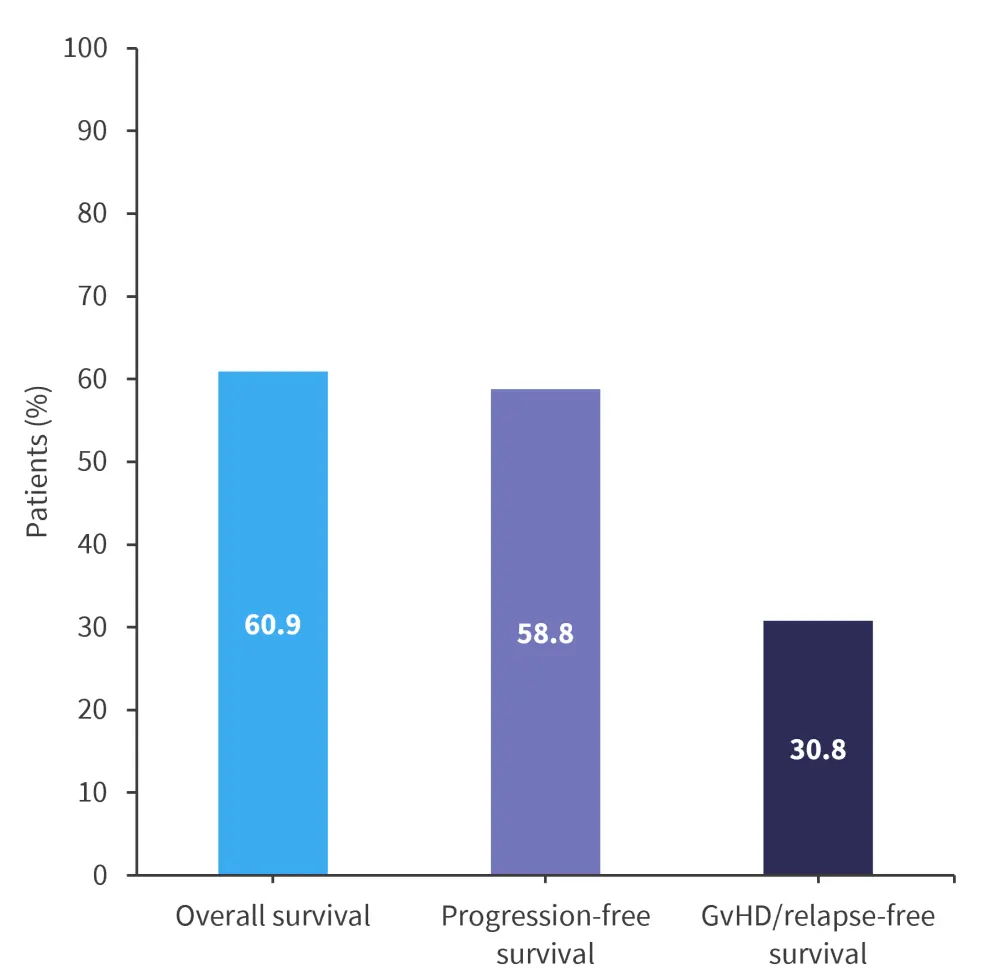
*Adapted from Ding, et al.1
A patient age of ≥18 years was found to independently predict an unfavorable prognosis of progression-free, GvHD-relapse-free, and overall, survival. In addition, liver involvement was an independent factor associated with poor progression-free survival.
Conclusion
In this study of hUCB-MSC, the greatest response was seen in skin GvHD, while liver aGvHD had the lowest overall response rate. The overall survival of (60.9%) in this trial was higher compared with trials investigating BM-MSCs; however, GvHD relapse-free survival was lower (30.8%). Overall, there is a lack of robust responses in SR-aGvHD with hUCB-MSC treatment; however, the treatment was shown to be a safe and effective salvage therapy for patients with SR-aGvHD. Additional large-scale multicentre trials are needed in the future.1
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
Which consideration most strongly guides your decision to escalate therapy in SR-aGvHD?

