All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional.
The gvhd Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the gvhd Hub cannot guarantee the accuracy of translated content. The gvhd and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The GvHD Hub is an independent medical education platform, sponsored by Medac and supported through grants from Sanofi and Therakos. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View GvHD content recommended for you
Effect of fluticasone propionate donor cell conditioning on the incidence and severity of GvHD
Do you know... This study demonstrated that fluticasone treatment leads to a reduction in T-cell activation in mice models. The fluticasone group was shown to have a significant reduction in which cell type?
Hematopoietic stem cells (HSCs) have the capability to self-renew, differentiate into lineage blood cells, and engraft bone marrow from the blood. However, after hematopoietic stem cell transplant (HSCT), disease relapse can occur.1 Another complication of HSCT is graft-versus-host disease (GvHD), which can lead to high rates of morbidity and mortality.1 A key aim of HSCT is to increase the number of successfully grafted HSCs and reduce the incidence of GvHD.
This study examined the effects of donor cell conditioning with fluticasone propionate prior to allogeneic-HSCT on the incidence and severity of post-HSCT GvHD in mouse models.1
Method1
The effect of glucocorticoids on mice HSCs was measured by culturing enriched murine bone marrow with vaeying concentrations of fluticasone or dexamethasone. After 16 hours of culture, it was found that using fluticasone 3 nM was the optimal dose for upregulation of CXCR4 expression on HSCs, with no impact on HSC viability.
Then, the effect of fluticasone treatment was examined to ascertain whether it would improve HSC engraftment in vivo. HSCs were pretreated with fluticasone or vehicle control (dimethyl sulfoxide) and compared in a mouse model, as shown in Figure 1. Cells taken from mice with H-2b major histocompatibility complex (MHC) were transplanted into lethally irradiated Balb/c (H-2d MHC) mice.
Figure 1. Method of mice receiving mismatched allogeneic or syngeneic transplant*
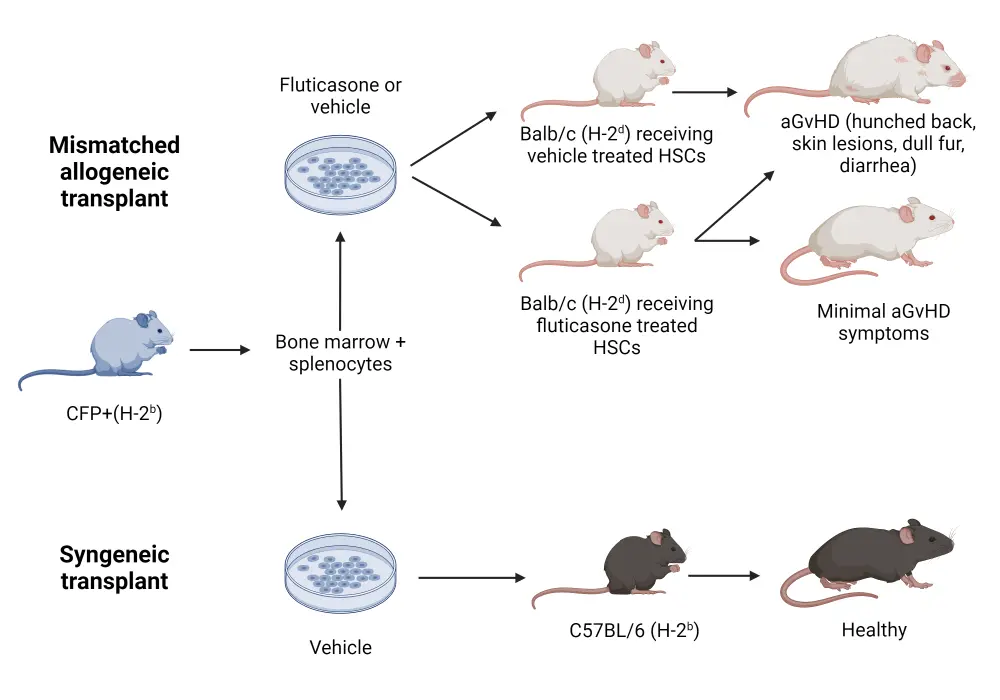
aGvHD, acute graft-versus-host disease; HSC, hematopoietic stem cell.
*Adapted from Varady, et al.1 Created with BioRender.com
Results1
In mice who received vehicle-treated cells, symptoms of acute graft-versus-host disease (aGvHD) were observed, such as weight loss and dull fur; all were euthanized by Day 27 posttransplant. In mice who received fluticasone-treated cells, half died due to GvHD and the rest displayed only minor GvHD symptoms, with a significantly lower overall clinical score compared to vehicle. A syngeneic control group was also investigated, demonstrating that all mice recovered quickly after treatment with vehicle-treated cells.
T-cell engraftment and activation
After 16 hours of culture, the fluticasone-treated group had a significant reduction in T- and B-cell lymphocyte viability compared with the vehicle group. However, at 7 days posttransplant, donor T-cell engraftment was observed in the blood, spleen, and liver of fluticasone and vehicle recipients. Therefore, it is suggested that the reduction in GvHD symptoms in fluticasone mice was not due to the absence of donor T-cell engraftment.
However, it is likely that fluticasone treatment leads to a reduction in T-cell activation. There was a significant decrease in CD69+ CD4 T cells and reduced CD44 expression in the blood, liver, and spleen of the fluticasone group compared with the vehicle group. In addition, in the fluticasone group, there was a significant reduction in Th1 effector cells, but no difference in Th2 and Th17 cells between the groups; therefore, Th1 response may be implicated in reducing GvHD severity in mice.
Secondary transplant with fluticasone-treated cells
To determine whether fluticasone-treated T cells retain long-term tolerance towards the host, Balb/c mice were transplanted with donor B6 cells treated with vehicle or fluticasone, before secondary transplants were performed in a second group of Balb/c mice; this is shown in Figure 2. Both groups of Balb/c mice were lethally irradiated pretransplant.
Figure 2. Secondary transplant of pretreated cells in Balb/c mice*
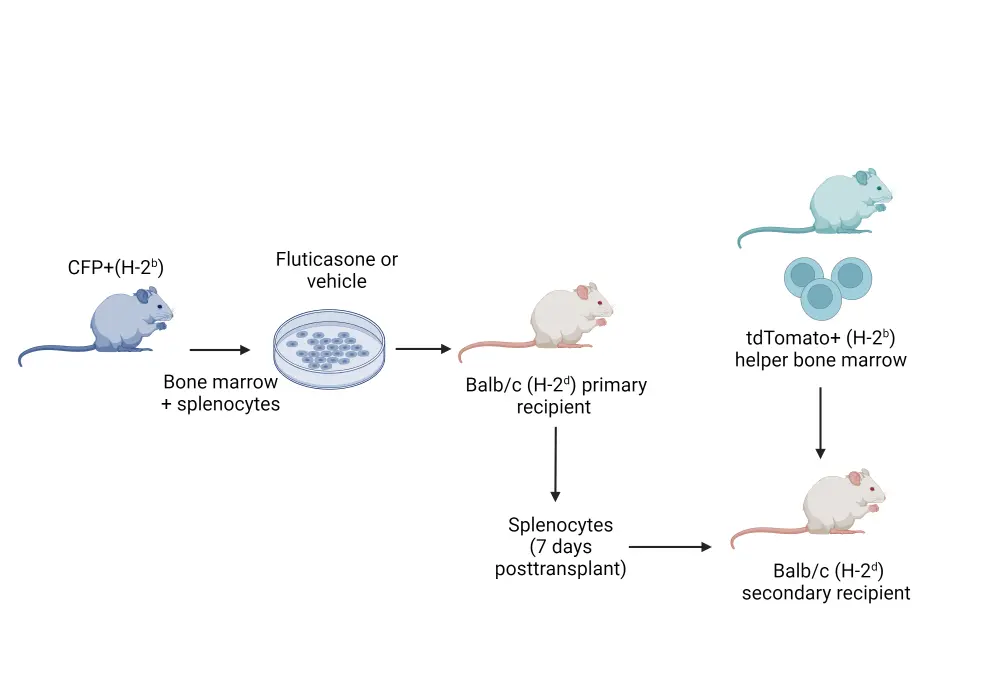
*Adapted from Varady, et al.1 Created with BioRender.com
In the vehicle group, less than 50% survived to Day 60 posttransplant, while all but one survived in the fluticasone group. The fluticasone group also displayed a significant reduction in the clinical grade of GvHD. The majority of T cells in the recipient’s blood were found to be tdTomato+ from the transplanted bone marrow, suggesting that cells from the original spleen culture did not expand in the secondary group of mice. Therefore, treatment with fluticasone may permanently deplete allogeneic T cells, or lead to long-lasting tolerance.
Conclusion
These mouse models have demonstrated that pretreatment with fluticasone before transplant can significantly reduce the incidence and severity of GvHD.1 There was evidence of T-cell expansion alongside a reduction in T-cell activation in fluticasone mice, suggesting a depletion of allogeneic T cells or tolerance.1 Fluticasone is commonly used in nasal sprays, and corticosteroid therapy is a first-line treatment for aGvHD after transplant; however, there are harmful side effects associated with long-term glucocorticoid treatment. Therefore, there may be a benefit to using fluticasone to treat donor cells rather than patients, thus avoiding potential side effects. Further human studies are needed to evaluate the efficacy of fluticasone in donor cell pre-treatment prior to transplant in GvHD prophylaxis.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
Which consideration most strongly guides your decision to escalate therapy in SR-aGvHD?

