All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional.
The gvhd Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the gvhd Hub cannot guarantee the accuracy of translated content. The gvhd and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The GvHD Hub is an independent medical education platform, sponsored by Medac and supported through grants from Sanofi and Therakos. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View GvHD content recommended for you
Dual T cell depletion for GvHD prophylaxis in adults post-haplo-HCT
The use of peripheral blood stem cells in haploidentical hematopoietic cell transplantation (haplo-HCT) has been associated with increased incidence of acute and chronic graft-versus-host disease (GvHD), 1 Posttransplant cyclophosphamide (PTCy) combined with antithymocyte globulin (ATG), and cyclosporine (CsA) has been used to prevent GvHD in a haplo-HCT setting.1
Moya et al.1 published a study in Bone Marrow Transplantation sharing real-world experience of ATG-PTCy-CsA in peripheral blood haplo-HCT. Here, we summarize the key findings.
Study design1
- A retrospective study of clinical data from adult patients who received peripheral blood haplo-HCT between October 2015 and December 2021 at Princess Margaret Cancer Centre, Toronto, Canada.
- Patients received dual T-cell depletion with ATG and PTCy at a dose of 50 mg/kg/day for 2 days followed by CsA 2.5 mg/kg intravenously every 12 hours from Day +5
- Patients received either 4.5 mg/kg or 2 mg/kg of ATG
- CsA was titrated to a therapeutic level ranging between 200 and 300 ng/ml and maintained therapeutic until Day +60 in the absence of GvHD
- To determine the outcomes of ATG-PTCy-CsA for haplo-HCT performed from peripheral blood stem cell grafts, the following were measured:
- cumulative incidence of Grade 2–4 and Grade 3–4 acute GvHD at Day 100;
- cumulative incidence of moderate to severe chronic GvHD at 1 and 2 years; and
- overall survival and non-relapse mortality at 1 and 2 years.
Key findings1
- In total, 157 patients were included in this study, with a median age of 57 years.
- Overall survival was 57.4% (95% confidence interval [CI], 48.8─65.1%) at 1-year posttransplant and 49.4% (95% CI, 40.4─57.7%) at 2-years posttransplant.
- Non-relapse mortality was 31.6% (95% CI, 24.2─39.2%) and 36.6% (95% CI, 28.5─44.7%) at 1 and 2 years, respectively. Figure 1 summarizes other posttransplant outcomes.
Figure 1. Outcome variables in patients who received ATG-PTCy-CsA prophylaxis*
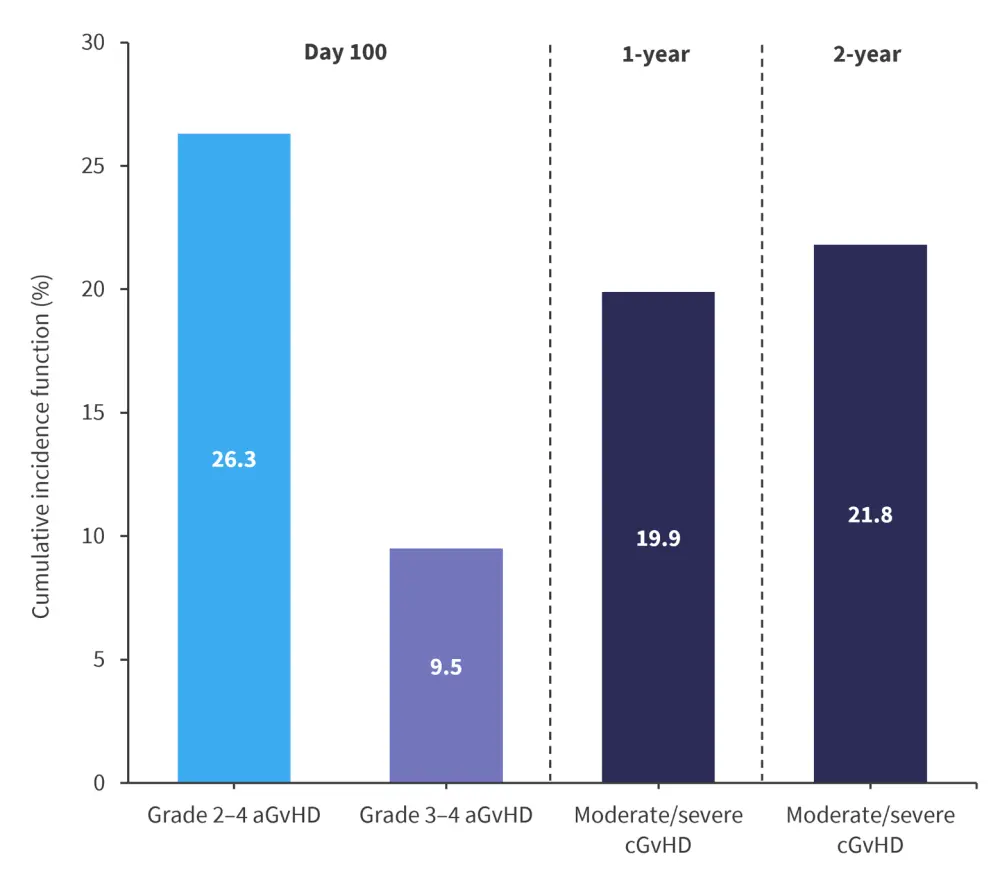
aGVHD acute graft-versus-host disease; ATG, antithymocyte globulin; cGvHD, chronic GvHD; CsA, cyclosporine; PTCy, posttransplant cyclophosphamide.
*Data from Moya, et al.1
- The administration of 2 mg/kg of ATG (p = 0.003) and the selection of donors older than 40 years (p = 0.011) increased the risk of Grade 3–4 acute GvHD.
- Overall, 46.5% of patients died with the leading causes of death being infections and relapse.
|
Key learnings |
|
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
Which consideration most strongly guides your decision to escalate therapy in SR-aGvHD?


