All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional.
The gvhd Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the gvhd Hub cannot guarantee the accuracy of translated content. The gvhd and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The GvHD Hub is an independent medical education platform, sponsored by Medac and supported through grants from Sanofi and Therakos. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View GvHD content recommended for you
Developments in GvHD prophylaxis in allogenic stem cell transplantation including the role of PTCy
Allogeneic hematopoietic stem cell transplantation (allo-HSCT) is a potentially curative therapy for many hematologic malignancies. However, graft-versus-host-disease (GvHD) remains a major limitation following allo-HSCT and a leading cause of morbidity and mortality. At the European Hematology Association (EHA) 2021 Virtual Congress, Luznik presented an educational session on the prevention of GvHD in allo-HSCT,1 specifically focusing on ex vivo T cell depletion (TCD) strategies and the latest clinical developments using posttransplant cyclophosphamide (PTCy).
GvHD prophylaxis
Pharmacological
The standard of care for GvHD prophylaxis is pharmacological modulation using methotrexate (MTX) plus calcineurin inhibitors (CNI). MTX-CNI is very effective in preventing acute GvHD (aGvHD) but not as effective in preventing chronic GvHD (cGvHD). In addition, while MTX-CNI is effective in preventing GvHD in human leukocyte antigen (HLA) matched sibling or 10/10 HLA-matched unrelated donor transplants, it is not effective for HLA-mismatched allografting.
T cell depletion
Various approaches have been used for TCD. Ex vivo graft manipulation with CD34 selection and in vivo graft manipulation with anti-thymocyte globulin (ATG) or alemtuzumab are effective strategies in preventing aGvHD and cGvHD. While extensive T cell removal with ATG and alemtuzumab effectively prevents GvHD, it also increases the risk of relapse, engraftment failure and infections.
Another in vivo strategy is the use of PTCy, which has also been shown to be effective in preventing aGvHD and cGvHD. First used for HLA-haploidentical transplant, PTCy has progressed to a variety of donor types and is becoming more widely adopted (Figure 1).
Progress in HLA-mismatched HSCT
Luznik highlighted the advances that have been made in GvHD prophylaxis for HLA-mismatched HSCT utilizing ex vivo and in vivo TCD strategies. Furthermore, there has been increasing use of PTCy in the last decade, as demonstrated by Phelan, et al.2 at the Center for International Blood and Marrow Transplant Research (Figure 1).
Figure 1. Haploidentical HCT and GvHD prophylaxis in the US*
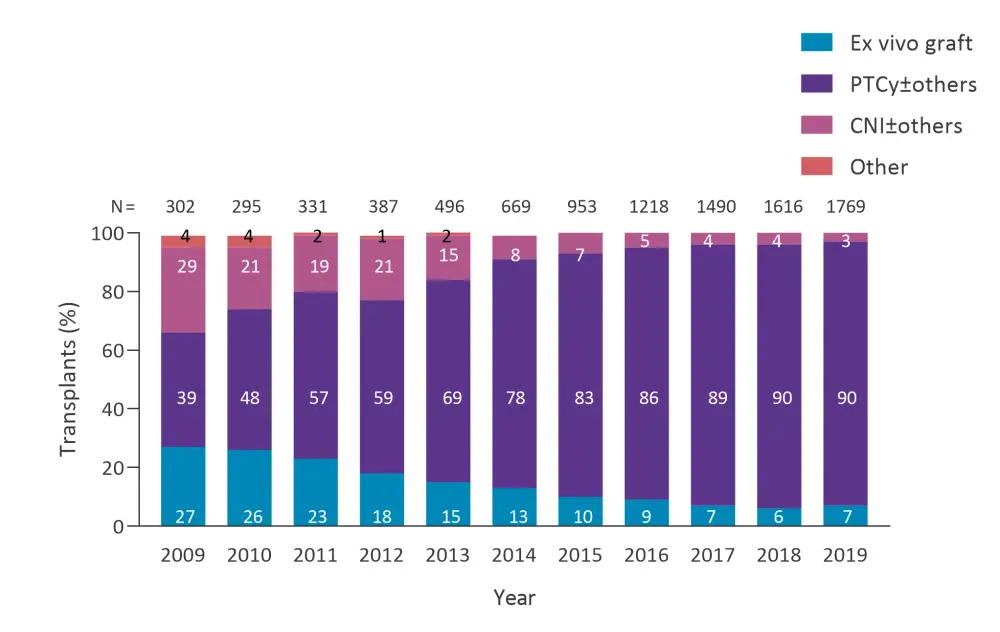
CNI, calcineurin inhibitor; HCT, hemopoietic cell transplantation; PTCy, posttransplant cyclophosphamide.
*Adapted from Phelan, et al.2
Luznik went on to highlight several studies that demonstrate PTCy as effective across different donor types. A study by Fuchs, et al.3 investigated reduced intensity conditioning and transplantation of double unrelated umbilical cord blood versus HLA-haploidentical related bone marrow for patients with hematologic malignancies. GvHd prophylaxis for double unrelated umbilical cord blood transplantation was cyclosporine and mycophenolate mofetil (MMF) and for haploidentical transplantation, PTCy, tacrolimus (TAC), and MMF. The findings established that there were no differences in outcomes for progression free survival, cumulative incidence of relapse, aGvHD Grade 2–4 or Grade 3–4, or cGvHD. However, transplant-related mortality was reduced and overall survival increased in the HLA-haploidentical related arm, who had received PTCy. Haploidentical transplantation has become, with the advent of PTCy, one of the most used alternative donor strategies.
In a recent retrospective review by Holton, et al.4 PTCy was shown to be effective in reducing GvHD compared with MTX/CNI for matched-unrelated donor (MUD). This highlights that an in vivo TCD strategy using PTCy overcomes the HLA mismatch barrier without impacting the graft-versus-leukemia effect.
Bolanos-Meade, et al.5 published a phase II trial in The Lancet Hematology, evaluating either maraviroc, bortezomib or PTCy compared with controls receiving a combination of TAC and methotrexate. The trial showed that the composite endpoint, GvHD-free relapse-free survival, cumulative incidence of Grade 3–4 GvHD, and cGvHD requiring systemic immunosuppression was better with PTCy, MMF and TAC. Luznik went on to state that further trials are required to confirm the efficacy of PTCy, MMF, and TAC. In addition, further trials of PTCy vs ATG are warranted following several retrospective studies that have suggested non-inferiority.
Data from an ongoing phase III trial of TAC/MTX vs CD34+ TCD vs PTCy in HLA-matched HSCT have not shown any difference in the primary endpoint of cGvHD relapse-free survival.6,7 Ex vivo TCD was associated with reduced frequency of Grade 2–4 aGvHD, but the highest rate of infection. PTCy was associated with an increased incidence of Grade 2–3 infections but had the lowest rates of relapse compared to TAC/MTX and ex vivo TCD. Interestingly, overall survival was similar in PTCy and TAC/MTX but worse in ex vivo TCD suggesting the increased incidence of infections with PTCy, when compared with TAC/MTX and ex vivo TCD, was not reflected in the overall survival.1
Variables predictive of PTCy GvHD
Luznik went on to discuss developments in the understanding of the mechanism of PTCy in GvHD prophylaxis and identifying the markers of GvHD including pretransplant factors, immune subsets, serum markers, and proteomic markers from machine learning.
In a comparison of two cohorts of patients who had received myeloablative conditioning regimen (MAC) haploidentical-HSCT followed by PTCy, MMF and TAC (n = 70), or MAC HLA matched-HSCT followed by PTCy (n = 74), Luznik, et al.7 analyzed 10 pre-transplant factors, 18 immune subsets, and seven serum markers using flow cytometry, proteomics and transcriptomics.
Use of unbiased machine-based learning revealed two variables—interferon-based cytokine CXCL-9 and CD4+ conventional T (Tconv) cells—which were associated with aGvHD. The presence of these markers was associated with a higher incidence of aGvHD, with Tconv regulation involved in many of these incidences. Flow cytometry further confirmed that patients with aGvHD expressed CXCR3, the ligand for CXCL-9, suggesting that high Tconv and CXCL-9 levels after HSCT were predictive of aGvHD.
Further analyses compared samples from patients with and without aGvHD before the development of aGvHD. Assessing effector T-cell associated transcriptional activation markers in these samples found an upregulation of these in Tconv cells. Further analysis found that amongst the cell phenotypes exhausted, activated, or naïve, the most dominant was activated Tconv confirming upregulation of these cells before post PTCy acute GvHD onset. This suggests that aGvHD in PTCy treated patients is mostly modulated by Tconv cells.
Luznik, et al. went on to assess metabolic signatures in Tconv cells and found that GvHD appears to activate oxidative-phosphorylation more than glycolysis or fatty-acid metabolism, suggesting that PTCy may not only deplete T cells, but also modulate them and that an awareness of this may improve understanding of drug tolerance and how to treat aGvHD in these patients.
A subsequent question addressed by Luznik, et al., was about regulatory T cells (Treg) which seem to persist despite PTCy treatment. They found that the ratio of Treg to Tconv remained high irrespective of GvHD in matched or haploidentical. In addition, they found distinct transcriptional signatures in Treg enriched samples that differentiated patients who developed GvHD from those who did not (IFNG, GZMA, GZMK). This was further supported by genetic analysis, which showed positive enrichment of genetic signatures associated with working Treg cells in patients who develop GvHD.
Conclusion
The presentation summarized the value of PTCy in different types of donors such as MUD, haploid, and mismatched-MUD. PTCy can be combined with ATG in hematologic malignancies but needs further investigation. Activation status and metabolic signatures of Tconv cells are risk markers for aGvHD post PTCy. Furthermore, PTCy offers a unique opportunity for GvHD prophylaxis combinations using immune predictive variables.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
Which consideration most strongly guides your decision to escalate therapy in SR-aGvHD?

