All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional.
The gvhd Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the gvhd Hub cannot guarantee the accuracy of translated content. The gvhd and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The GvHD Hub is an independent medical education platform, sponsored by Medac and supported through grants from Sanofi and Therakos. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View GvHD content recommended for you
Baricitinib use in patients with aGvHD receiving HLA-matched PBSCT
Janus kinase (JAK) inhibitors, such as baricitinib and ruxolitinib, are effective in treating steroid-refractory graft-versus-host disease (GvHD).1 However, up to 50% of patients become refractory to ruxolitinib; therefore, alternative JAK inhibitors are needed.1
At the 64th American Society of Hematology (ASH) Annual Meeting and Exposition, Schroeder1 presented results from a phase I, open-label trial of baricitinib for the treatment of acute (a)GvHD, which we are pleased to summarize here. Baricitinib has been shown to be most effective in preventing GvHD when used early after transplant.
Study design
A total of 24 adult patients were enrolled to receive baricitinib in two dosing cohorts (2 mg or 4 mg, with 12 patients in each). The study design is shown in Figure 1. To be included in the study, patients had to be aged >18 years, diagnosed with a hematologic malignancy, and received a transplant from a human leukocyte antigen-matched peripheral blood stem cell donor. The primary objectives were graft failure and incidence of aGvHD by Day 100, with a secondary objective of treatment-related mortality at Day 180.
Figure 1. Study design*
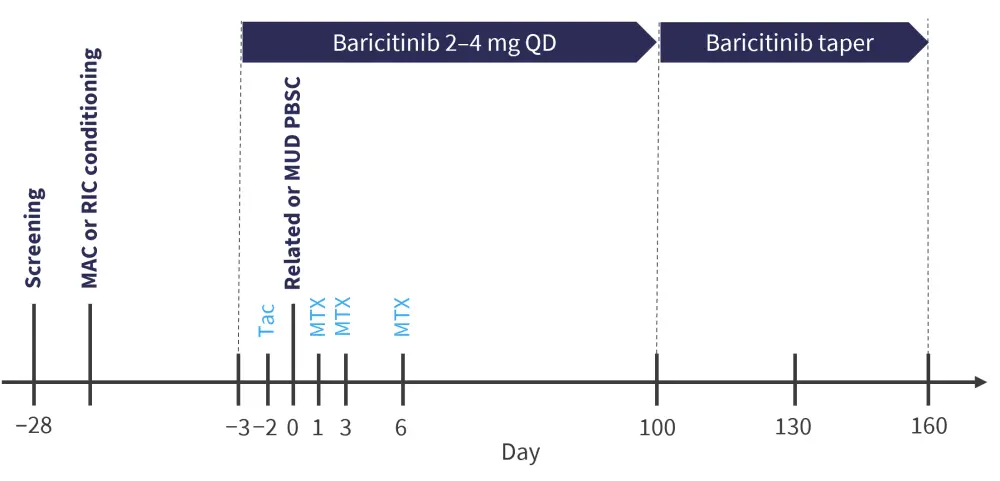
MAC, myeloablative conditioning; MTX, methotrexate; MUD, matched unrelated donor; PBSC, peripheral blood stem cells; RIC, reduced intensity conditioning, Tac, tacrolimus.
*Adapted from Schroeder.1
Results
Baseline patient characteristics are shown in Table 1. The majority of patients had a diagnosis of acute myeloid leukemia, and more patients in the 2 mg cohort compared with the 4 mg group were minimal residual disease negative at the start of the study (83.3% vs 41.6%, respectively).
Table 1. Baseline patient characteristics*
|
ALL, acute lymphoblastic leukemia; AML, acute myeloid leukemia; GvHD, graft-versus-host disease; MAC, myeloablative conditioning; MDS, myelodysplastic syndromes; MMF, mycophenolate mofetil; MRD+, minimal residual disease positive; MRD−, minimal residual disease negative; MTX, methotrexate; MUD, matched unrelated donor; PTCy, post-transplant cyclophosphamide; RIC, reduced intensity conditioning; Tac, tacrolimus; Thymo, thymoglobulin. |
|||
|
Characteristic, number of patients (unless otherwise stated) |
2 mg baricitinib |
4 mg baricitinib |
Total |
|---|---|---|---|
|
Age, years |
28–71 |
42–72 |
28–72 |
|
Diagnosis |
|
|
|
|
AML |
8 |
8 |
16 |
|
MDS |
2 |
4 |
6 |
|
ALL |
2 |
0 |
2 |
|
Remission status at transplant† |
|
|
|
|
MRD+ |
2 |
6 |
8 |
|
MRD− |
10 |
5 |
15 |
|
Donor source |
|
|
|
|
Sibling |
6 |
4 |
10 |
|
MUD |
6 |
8 |
14 |
|
Conditioning |
|
|
|
|
MAC |
11 |
8 |
19 |
|
RIC |
1 |
4 |
5 |
|
GvHD prophylaxis |
|
|
|
|
Tac/MTX |
12 |
7 |
19 |
|
PTCy/Tac/MMF |
0 |
5 |
5 |
|
Thymo added for MUD |
3 |
2 |
5 |
Safety
Adverse events of interest are shown in Table 2. Mucositis was the most common adverse event and occurred in 41.3% of patients. There were more patients in the 4 mg cohort who had thrombosis compared with the 2 mg cohort (three patients vs one patient, respectively).
Table 2. Adverse events of interest*
|
SAE, serious adverse event. †One case of intracranial hemorrhage at 2 mg and one case of pneumonia in the 4 mg baricitinib group, but both occurred after discontinuation of baricitinib. |
|
|
Adverse event, number of patients |
Overall |
|---|---|
|
Non-hematologic Grade 3–4 SAEs |
|
|
Mucositis |
10 |
|
Hypertension |
7 |
|
Febrile neutropenia |
7 |
|
Infections/sepsis |
7 |
|
Anorexia |
5 |
|
Diarrhea |
4 |
|
Thrombosis |
4 |
|
BK polyomavirus cystitis |
2 |
|
Deep vein thrombosis |
4 |
|
Death |
2† |
Efficacy
There was a median follow-up of 320 days (range, 63–368 days). Outcome measures are shown in Figure 2. A total of 20 patients were evaluable for engraftment at Day 100, and there were no cases of graft failure in either dosing cohort. There were two patients in the 2 mg cohort and three patients in the 4 mg cohort who relapsed at Day 300. The overall survival at 1 year was 71%. Chronic GvHD was recorded in nine patients (three mild cases and six moderate). In total, eight cases of Grade 2–4 GvHD were recorded, with 25% being steroid refractory, while all cases of Grade 2 aGvHD were steroid responsive.
Figure 2. Outcome measures*
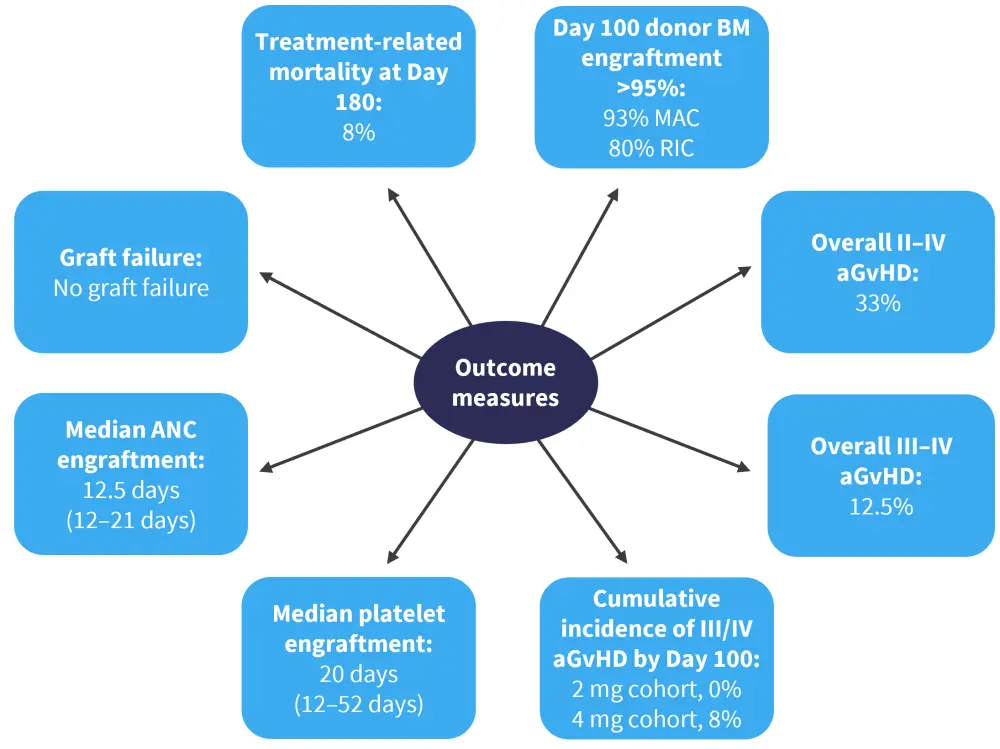
aGvHD, acute graft-versus-host disease; ANC, absolute neutrophil count; BM, bone marrow; MAC, myeloablative conditioning; RIC, reduced intensity conditioning.
*Adapted from Schroeder.1
Conclusion
This study demonstrates that both 2 mg and 4 mg baricitinib dosing regimens can be given effectively to patients who have undergone peripheral blood stem cell transplantation. There was a low incidence of Grade 3 and 4 aGvHD, with an acceptable safety profile, although cases of thrombosis were higher in the 4 mg-treated patients. There were no cases of graft failure in either cohort, with higher bone marrow engraftment seen in patients who had received myeloablative conditioning compared with reduced intensity conditioning. There is an expansion planned to use the 2 mg dose of baricitinib in patients receiving myeloablative conditioning.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
Which consideration most strongly guides your decision to escalate therapy in SR-aGvHD?


