All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional.
The gvhd Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the gvhd Hub cannot guarantee the accuracy of translated content. The gvhd and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The GvHD Hub is an independent medical education platform, sponsored by Medac and supported through grants from Sanofi and Therakos. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View GvHD content recommended for you
Novel drugs for treating cGvHD: preliminary phase I/II results for baricitinib
Chronic graft-versus-host disease (cGvHD) is associated with organ function impairment, shorter survival, and immune suppression, affecting multiple organ systems. It is considered a leading cause of morbidity and late nonrelapse mortality following allogeneic hematopoietic cell transplantation (allo-HCT). Therapeutic options are limited for the treatment of patients with refractory cGvHD, and therefore represent an unmet medical need.1
During the 62nd American Society of Hematology (ASH) Annual Meeting and Exposition, Noa G. Holtzman presented early phase I/II results for oral baricitinib, a novel Janus kinase (JAK) inhibitor, which is currently being investigated for the treatment of patients with therapy-refractory moderate to severe cGvHD following allo-HCT.1 Here, we are pleased to summarize key points.
Baricitinib is a JAK 1/2 inhibitor approved by the U.S. Food and Drug Administration for the treatment of adults with moderately-to-severely active rheumatoid arthritis.2
Study design
This was a single arm, intrapatient dose escalation study investigating the efficacy and safety of oral baricitinib at a daily dose of 2 mg (NCT02759731). The basis for selecting baricitinib as a potential therapy in cGvHD was associated with the key role of JAK-STAT pathway T-cell proliferation, and immune regulation, and the therapeutic efficacy in GvHD shown with another JAK inhibitor, ruxolitinib.
Safety and tolerability endpoints were adverse event (AE) rate, severity and duration, and dose-limiting toxicity (DLT) assessment on Day 28, at each dose level. Primary efficacy endpoint was overall response rate (ORR), defined as complete response (CR) plus partial response (PR) at 6 months, per 2014 National Institutes of Health (NIH) cGvHD Response Criteria. Patients with PR or stable disease (SD) were treated for up to 12 months and monitored for 12 months after treatment was completed.
Dose levels included 1 mg, 2 mg, and 4 mg daily, and the starting dose was 2 mg. The study design is shown in Figure 1.
Figure 1. Study design
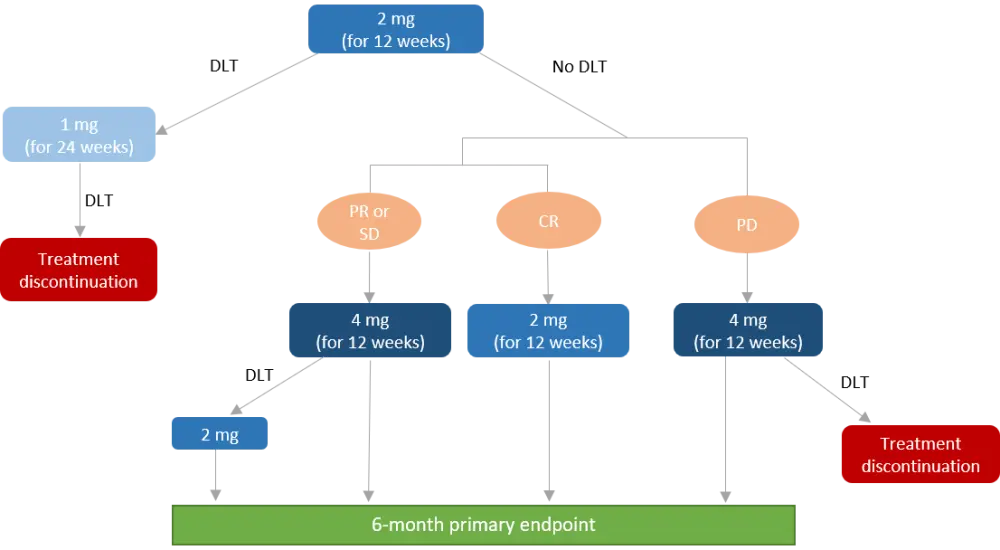
CR, complete response; DLT, dose-limiting toxicity; PD, progressed disease; PR, partial response; SD, stable disease.
Baseline characteristics
The total number of patients was 20 with a median age of 54 years (range 24–68 years). Median Karnofsky performance status was 80% (range, 80–90%). The vast majority of patients received a transplant using peripheral blood stem cells (95%) for treating their underlying disease (65% acute leukemias).
All patients had severe cGvHD per NIH criteria with three or more organs affected in 75%, and 90% of patients had sclerotic skin cGvHD. Patients had received a median of four prior therapies and 85% were on immunosuppressive therapy at time of enrollment.
Results
Safety and tolerability
No DLT events were observed, and 80% of patients had their doses escalated to 4 mg at 3 months. Most patients (95%) experienced AEs and the rate of possible treatment-related AEs was 85%. The rate of Grade 3 or higher AEs and severe AEs was 65% and 30%, respectively. 30% of Grade 3 AEs were considered as possibly treatment related.
There were no fungal or mycobacterial infections and most viral infectious complications (CMV reactivation, Epstein-Barr viremia, BK viruria) were self-limited, not requiring therapy. Results of the safety analysis are summarized in Table 1.
There were no deaths, or relapsed malignancy. Dose reductions and dose interruptions occurred in 15% and 55% of patients, respectively. Early study discontinuation for toxicity, disease progression, and other reasons (n = 3, each) occurred in 45% of patients with a median time to discontinuation of 5.6 months (range 0.8–10.7).
Table 1. Safety outcomes1
|
AE, adverse event; FEV1, forced expiratory volume. *Possibly treatment-related AE. |
|
|
Grade 3–4 AEs, n |
N = 20 |
|---|---|
|
Hypophosphatemia* Hypokalemia Pneumonia* Decreased FEV1 Skin infection* Hypertension Pain in extremity Neutropenia* Upper respiratory infection* |
5 4 3 3 (Grade 3, n = 2; Grade 4, n = 1) 2 1 1 1 1 |
Efficacy
- ORR at 6-months: 65% (95% CI, 50–85%) with best ORR at any time: 90% (95% CI, 85–100%)
- Median time to best response: 1.4 months (range, 1.4–6.3 months)
- Durable responses seen in 8/9 evaluable patients at 12 months with three patients progressing following treatment discontinuation
- Organ-specific response rates:
- Lower gastrointestinal: 100%
- Joints/fascia: 85%
- Mouth: 50%
- Lungs: 10%
- Steroid dose could be tapered in those on steroid at the time of enrollment
- Clinically meaningful improvements were observed in Lee Symptom Scale
- Long-term follow-up results (median 24.2 months):
- Median failure-free survival: 20.6 months (range, 19–not reached)
- Relapsed malignancy: 10%
- Overall survival: 100%
Conclusion
This study included patients with severe cGvHD who received multiple lines of therapy. Baricitinib has shown an acceptable safety profile and was considered well tolerated. The response rate was 65% at 6 months, and rapid, durable responses were reported in different organs. This study is still ongoing, and full results are expected in 2022.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
Which consideration most strongly guides your decision to escalate therapy in SR-aGvHD?

