All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional.
The gvhd Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the gvhd Hub cannot guarantee the accuracy of translated content. The gvhd and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The GvHD Hub is an independent medical education platform, sponsored by Medac and supported through grants from Sanofi and Therakos. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View GvHD content recommended for you
An overview of the role of ATG in preventing acute and chronic GvHD after allo-HSCT
Graft-versus-host disease (GvHD) can significantly impact the morbidity, mortality, and quality of life (QoL) of allogeneic hematopoietic stem cell transplantation (allo-HSCT) recipients. The immunologic graft-versus-host response that precedes acute GvHD (aGvHD) occurs within hours following HSCT, long before the disease is clinically evident. Chronic GvHD (cGvHD) arises in the days after HSCT and can be challenging to manage without compromising the graft-versus-leukemia (GvL) effect.
Rabbit antithymocyte globulin (ATG) has proven to be an effective prophylaxis for the prevention of GvHD, however, intensified immunosuppressive treatment may cause serious side effects and therefore, it is suggested only for high-risk patients. Despite this, only a few studies have touched upon ATG dosing protocols and its effects on relapse and infection, especially so in the case of HLA-matched sibling donor transplantations (MSD-T).
Two recent studies have assessed the impact of ATG on high-risk patients1 and on patients who have undergone MSD-T.2
Khanolkar et al. examined whether GvHD could be reduced in patients with high serum soluble interleukin-2 receptor alpha (sIL2Ra) or low interleukin-15 (IL-15) on Day 7 by pre-emptively administering ATG on Day 8.1 The two biomarkers indicate a high risk of developing clinically significant GvHD (sGvHD), defined as Grade 2−4 aGvHD or moderate to severe cGvHD.
Cho et al. investigated the ability of low-dose ATG to prevent cGvHD for patients with acute leukemia who underwent MSD-T.2
Study by Khanolkar et al. on biomarker-guided, prophylactic ATG therapy1
Study design
This was a phase II, prospective, open-label, non-randomized, single-center trial. A diagram depicting the study design is shown in Figure 1.
The HSCT conditioning regimen comprised of intravenous (IV) fludarabine (50 mg/m2/day on Day −6 to Day −2), IV busulfan (3.2 mg/kg/day on Day −5 to Day −2), and total body irradiation (TBI) (4 Gy in two fractions on Day −1 and Day 0). GvHD prophylaxis included IV ATG (0.5 mg/kg on Day −2, 2.0 mg/kg on Day −1, and 2.0 mg/kg on Day 0) and methotrexate on Day 1, Day 3, Day 6, and Day 11 and cyclosporine from Day −1 to 84.
Figure 1. Study flowchart*
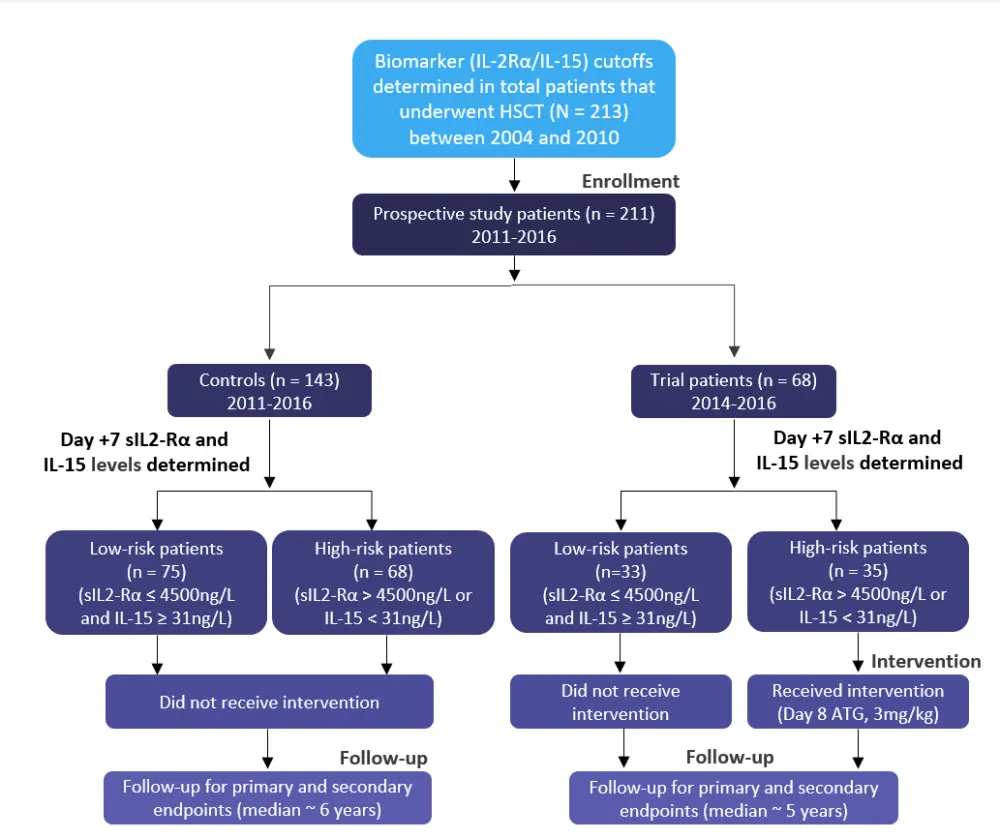
ATG, antithymocyte globulin; HSCT, hematopoietic stem cell transplantation; IL-15, interleukin-15; sIL2Ra, serum soluble interleukin-2 receptor alpha.
*Adapted by Khanolkar et al.1
- Major inclusion criteria were:
- Age ≥ 18 years
- Myeloablative conditioning (MAC)
- Filgrastim-mobilized blood stem cell graft
- GvHD prophylaxis with ATG (4.5 mg/kg given between Day −2 and 0), methotrexate and, cyclosporine
- Primary endpoint was the cumulative incidence of sGvHD.
- Secondary endpoints included:
- Overall survival (OS)
- Nonrelapse mortality (NRM)
- Cumulative incidence of relapse (CIR)
Both study arms were well balanced in their characteristics (Table 1).
Table 1. Selected patient characteristics*
|
ATG, antithymocyte globulin; Bup, busulfan; Cy, cyclophosphamide; Flu, fludarabine; N/A, not applicable; PBSCs, peripheral blood stem cells; TBI, total body irradiation. |
|||||||
|
Characteristic, % |
Study arm |
Control arm |
p value† |
||||
|---|---|---|---|---|---|---|---|
|
Low-risk patients |
High-risk patients |
Total |
Low-risk patients |
High-risk patients |
Total |
||
|
Patient age, years, median (range) |
53 |
53 |
53 |
48 |
53 |
49 |
0.171 |
|
Donor age, years, median (range) |
30 |
31 |
32 |
34 |
31 |
30 |
0.577 |
|
Graft type |
N/A |
||||||
|
PBSCs |
100 |
100 |
100 |
100 |
100 |
100 |
|
|
Conditioning |
1.000 |
||||||
|
Flu-Bup-ATG-TBI |
100 |
97 |
99 |
97 |
100 |
99 |
|
|
Flu-Cy-ATG-TBI 2 |
0 |
3 |
1 |
3 |
0 |
1 |
|
|
Disease risk |
0.656 |
||||||
|
Low/intermediate |
94 |
79 |
87 |
85 |
83 |
84 |
|
|
High/very high |
6 |
15 |
10 |
9 |
17 |
13 |
|
|
Unknown |
0 |
6 |
3 |
5 |
0 |
3 |
|
|
Median follow-up, days (range) |
1451 (53−2257) |
1389 (53−2257) |
1455 (53−2257) |
1783 (24−3266) |
1654 (27−3189) |
1743 (24−3266) |
0.003 |
|
Median follow-up of surviving patients, days (range) |
1813 (1033−2257) |
1611 (1364−2257) |
1839 (1033−2257) |
2261 (141−3266) |
2250 (89−3189) |
2250 (89−3266) |
< 0.001 |
Results
- The prevalence of clinically sGvHD was significantly lower in high-risk patients who had Day 8 ATG compared with high-risk controls (subdistribution hazard ratio [SHR] = 0.48; p = 0.045).
- There was no statistically significant difference in OS between low-risk (hazard ratio [HR] = 0.97; p = 0.971) or high-risk (HR = 1.66; p = 0.101) study patients and controls.
- High-risk study patients had a significantly higher incidence of non-GvHD-associated NRM compared with high-risk controls (SHR = 3.73; p = 0.032), usually related to infection
- Despite the significant decrease in sGvHD incidence in the high-risk study patients compared with the high-risk controls, no significant difference was found in GvHD-associated NRM, due to Day 8 ATG reducing Grade 2 aGvHD, which is usually non-fatal, but not Grade 3−4 aGvHD or moderate to severe cGvHD.
- There was no significant difference between high-risk trial patients and high-risk controls in CIR.
- When comparing high- and low-risk controls, no significant difference was found in the incidence of sGvHD (SHR = 1.18; p = 0.519). This suggested that sIL-2Ra and IL-15 levels no longer effectively stratified sGvHD risk in the 2011−2016 patient cohort even though they had predicted a 2.5-fold difference in sGvHD incidence in the 2004−2010 group.
Study by Cho et al. on low-dose ATG in MSD-T setting2
Study design
A prospective, single-center, open-label, randomized, phase III study of 120 adult patients randomly assigned to receive or not receive ATG (1.25 mg/kg/day) on Days −3 and −2 was carried out.
Taking into account age and/or comorbidities, patients were treated with either MAC or reduced-intensity conditioning (RIC) regimens.
- MAC: Cyclophosphamide (120 mg/kg) combined with 1,320 cGy of fractionated TBI or busulfex (12.8 mg/kg).
- RIC: Busulfex (6.4 mg/kg) and fludarabine (150 mg/m2) with 400 cGy of fractionated TBI or busulfex (9.6 mg/kg) and fludarabine (150 mg/m2).
All patients received GvHD prophylaxis with cyclosporine (target serum trough level of 150−300 ng/mL) and methotrexate (10 mg/m2 on Days 1, 3, 6, and 11).
- Primary endpoint was the cumulative incidence of cGvHD at 2 years according to the National Institutes of Health (NIH) criteria.
- Secondary endpoints included:
- Engraftment
- Immune reconstitution
- Cumulative incidence of aGvHD
- Infectious complications
- Relapse (CIR)
- NRM
- Disease-free survival (DFS)
- OS
- cGvHD-free and relapse-free survival (cGRFS)
- Recipient reported outcomes with the Short Form (36) Health Survey (SF-36) questionnaire
Patients were eligible if they were 19 to 65 years of age, had acute myeloid or lymphoblastic leukemia (AML/ALL) in complete morphologic remission (CR), and had an Eastern Cooperative Oncology Group (ECOG) performance score < 2. Selected clinical characteristics of the enrolled patients are presented in Table 2 below. There were no significant differences between the two groups other than gender.
Table 2. Patient characteristics by ATG use*
|
ALL, acute lymphoblastic leukemia; AML, acute myeloid leukemia; ATG, antithymocyte globulin; BU, busulfex; CY, cyclophosphamide; DRI, disease risk index; FLU, fludarabine; HSCT, hematopoietic stem cell transplantation; MAC, myeloablative conditioning; RIC, reduced-intensity conditioning; TBI, total body irradiation. |
|||
|
Characteristic, % |
ATG arm |
No ATG arm |
p value |
|---|---|---|---|
|
Age, recipient at allo-HSCT, median (range) |
47.5 |
44.5 |
0.362 |
|
Gender, recipient, male |
61.0 |
39.0 |
0.018 |
|
Refined DRI, high risk |
15.0 |
13.3 |
0.793 |
|
Cytogenetic risk, high risk |
45.0 |
41.7 |
0.713 |
|
Disease type |
1.000 |
||
|
AML |
55.0 |
55.0 |
|
|
ALL |
45.0 |
45.0 |
|
|
Conditioning regimen |
0.613 |
||
|
CY + TBI 1320 cGy |
38.3 |
41.7 |
|
|
CY + BU |
25.0 |
28.3 |
|
|
FLU + BU |
25.0 |
25.0 |
|
|
FLU + BU + TBI 400 cGy |
11.7 |
5.0 |
|
|
Conditioning intensity |
0.562 |
||
|
MAC |
63.3 |
70.0 |
|
|
RIC |
36.7 |
30.0 |
|
Results
Statistically significant study results are listed in Table 3.
Table 3. Treatment outcomes*
|
ATG, antithymocyte globulin; cGvHD, chronic graft-versus-host disease; CI, confidence interval; EBV, Epstein Barr virus; NIH, National Institutes of Health. |
|||
|
Characteristic, % (95% CI) |
ATG arm |
No ATG arm |
p value |
|---|---|---|---|
|
cGvHD at 2 years |
|||
|
Overall grade mild to severe |
25.0 |
65.4 |
<0.001 |
|
Overall grade moderate to severe |
15.0 |
45.3 |
0.001 |
|
Skin involvement† |
26.3 |
0 |
0.027 |
|
Infectious complications |
|||
|
EBV viral reactivation (≥1000 IU/mL) |
21.8 |
5.1 |
0.013 |
- Immune reconstitution:
- The ATG group had significantly fewer CD4 T-cells posttransplant compared with the non-ATG group.
- Acute and chronic GvHD:
- The difference in the cumulative incidence of cGvHD in the ATG and non-ATG groups remained significant when adjusted by refined disease risk index (DRI) and conditioning intensity.
- 33% of patients in the ATG group were free from immunosuppressive treatment at 12 months compared with 12% in the non-ATG group (p = 0.004). Median time to stop all immunosuppressive treatment without resumption was 5.3 months (range, 2.3−11.8 months) in the ATG group and 9.6 months (range, 4.3−17.8 months) in the non-ATG group.
- Toxicity:
- The Grade > 2 infusion reactions in the ATG group were 33%.
- NRM and relapse:
- Older age was the only significant factor associated with increased NRM (p = 0.035).
- The 2-year CIR was higher in the ATG group (20.0%; 95% CI: 11.0−31.0) than in the non-ATG group (9.3%; 95% CI: 3.3−19.0; p = 0.055), with risk differing according to cytogenetic subgroup (non-high-risk, 12.2% vs 9.2%, p = 0.596; high-risk, 29.6% vs 9.3%, p = 0.042).
- Refined DRI was the only significant factor associated with increased CIR (p = 0.038).
- Survival outcomes:
- Older age was the only significant factor associated with inferior OS (p = 0.024).
- Improvement in cGRFS was significant in the non-high-risk cytogenetic subgroup (ATG group 45.5% vs non-ATG group 0%; p = 0.038)
- Impact of absolute lymphocyte count (ALC) at the time of ATG administration:
- ATG use reduced cGvHD more efficiently in patients with Day −3 ALC ≤ 0.1 × 109/L (ATG group 66.7% vs non-ATG group 9.7%; p < 0.001) than patients with Day −3 ALC > 0.1 × 109/L (63.2% vs 41.4%; p= 0.261).
- cGRFS was significantly improved by ATG use in patients with Day −3 ALC ≤ 0.1 × 109/L (67.7% vs 18.0%; p = 0.002) with no difference in the −3 ALC > 0.1 × 109/L (24.1% vs 21.0%; p = 0.410).
- TBI conditioning had significant effects on ALC on Day −3 with 66.7% of patients who received TBI having an ALC ≤ 0.1 × 109/L compared with 31.8% of patients who received non-TBI regimens (p < 0.001).
- Multivariate analysis for the effects of ATG on cGvHD and cGRFS adjusted by TBI conditioning in each ALC group confirmed the benefit of ATG in patients with Day −3 ALC ≤ 0.1 × 109/L, whereas no benefit in patients with day −3 ALC > 0.1 × 109/L.
- QoL:
- At 12 months after allo-HSCT, the ATG group reported better scores for the physical components of the survey, including physical role and function, body pain, and general health.
Conclusion
Results from the first study showed that biomarker-guided, pre-emptive ATG therapy is achievable and effective at lowering sGvHD without increasing relapse, thus, improving the morbidity and poor QoL associated with GvHD. Though, the authors noted a heightened risk of fatal infections, and a lack of benefit in survival. The main limitation of the study was the lack of randomization. In the second study, low-dose ATG successfully reduced the incidence of cGvHD in MSD-T, resulting in improvement in QoL. This was more noticeable in patients with acute leukemia without adverse cytogenetic characteristics and with low absolute lymphocyte count on the first day of administration, in whom cGRFS was significantly superior. However, for patients with high-risk acute leukemia, treating physicians should ensure that the increased risk of relapse does not outweigh the benefit. Furthermore, low-dose ATG had no significant impact on aGvHD. Future randomized trials should focus on strategies to decrease the risk of infections associated with intensified GvHD prophylaxis, on personalized optimal ATG dosing and administration schedules, as well as more conclusively determining which patients may be better suited for intensified preventative GvHD treatment. The use of validated biomarkers may prove to be helpful in stratifying patients according to their risk of developing GvHD; hence allowing for tailored management approaches that inhibit adverse outcomes.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
Which consideration most strongly guides your decision to escalate therapy in SR-aGvHD?

